Welcome to the chemistry class science 10 chapter 1 – Chemical Reactions and Equations Short Notes. The short notes are prpaprd soloely from the NCERT books to make prepare well for the exam.
You can rely on the short notes below to make your preparation well enough to score full marks in the science paper.
Signs Of A Chemical Reaction
A chemical reaction has taken place if you observe:
- Change in state
- Change in colour
- Evolution of a gas
- Change in temperature

Chemical Equations
Word-Equation
A shorter way to describe a chemical reaction.
Example:
Burning Magnesium:
Magnesium + Oxygen → Magnesium Oxide

Components of a chemical reaction-
Reactants:
Substances that undergo chemical change (LHS)
Product:
The new substance formed (RHS)
Equation Format
- Reactants are written on the LHS with a plus sign (+) between them.
- The arrow (→) points towards the products.
- Products are written on the RHS with a plus sign (+) between them.
General Form:
Reactant 1 + Reactant 2 → Product
Writing a Chemical Equation
Chemical Formulae Method
A more concise way to represent reactions using chemical formulae instead of words.
Example
Burning Magnesium:
Mg + O₂ → MgO
Balanced vs. Unbalanced Equations
Skeletal Chemical Equation
- An equation where the number of atoms of each element is not the same on both sides (LHS and RHS)
- Mass is not the same on both sides of the equation
- Example:
Mg + O₂ → MgOis a skeletal chemical equation
Checking Balance
Count and compare the number of atoms of each element on:
- LHS (Left-Hand Side)
- RHS (Right-Hand Side)
If atom counts are equal on both sides → Balanced Equation
If atom counts are unequal → Unbalanced Equation (Skeletal Equation)
Balanced Chemical Equations
Law of Conservation of Mass
- Mass can neither be created nor destroyed in a chemical reaction
- Total mass of elements in products = Total mass of elements in reactants
- Number of atoms of each element remains the same before and after reaction
Balancing Chemical Equations
Example
Zinc + Sulphuric Acid
Zn + H₂SO₄ → ZnSO₄ + H₂
Atom Count
| Element | LHS | RHS |
|---|---|---|
| Zn | 1 | 1 |
| H | 2 | 2 |
| S | 1 | 1 |
| O | 4 | 4 |
Therefore, The above equation is balanced ✅
Balancing an Unbalanced Equation
Example
Iron + Water
Fe + H₂O → Fe₃O₄ + H₂
Step I: Count atoms
| Element | LHS | RHS |
|---|---|---|
| Fe | 1 | 3 |
| H | 2 | 2 |
| O | 1 | 4 |
Step II: Balance oxygen atoms
Put coefficient ‘4’ as 4H₂O (not H₂O₄)
Fe + 4H₂O → Fe₃O₄ + H₂
Step III: Balance hydrogen atoms
Make four hydrogen molecules on RHS
Fe + 4H₂O → Fe₃O₄ + 4H₂
Step IV: Balance iron atoms
Take three atoms of Fe on LHS
3Fe + 4H₂O → Fe₃O₄ + 4H₂
Step V: Final check
| Element | No. of atoms in reactants | No. of atoms in products |
|---|---|---|
| Fe | 3 | 3 |
| H | 8 | 8 |
| O | 4 | 4 |
✅ Equation is balanced
Physical States Notation
- (g) – gaseous
- (l) – liquid
- (aq) – aqueous (solution in water)
- (s) – solid
Example with physical states:
3Fe(s) + 4H₂O(g) → Fe₃O₄(s) + 4H₂(g)
Reaction Conditions
Indicated above/below the arrow
Examples:
CO(g) + 2H₂(g) 340 atm→ CH₃OH(l)
6CO₂(aq) + 12H₂O(l) → C₆H₁₂O₆(aq) + 6O₂ + 6H₂O
The above reaction takes place in the presence of chlorophyll and sunlight
TYPES OF CHEMICAL REACTIONS
During a chemical reaction:
- Atoms don’t change into other elements
- Atoms don’t disappear/appear
- Bonds break and form to make new substances
Combination Reaction
Definition of combination reaction
- Two or more reactants form a single product
- General form: A + B → AB
Examples:
1. CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat
CaO = Quick Lime
Ca(OH)₂ = Slacked Lime
2. C(s) + O₂(g) → CO₂(g)
3. 2H₂(g) + O₂(g) → 2H₂O(l)

Exothermic Reaction
Definition
- Heat is released with product formation
- Reaction mixture becomes warm
Examples of exothermic reactions:
1. CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
2. C₆H₁₂O₆ (aq) + 6O₂ (aq) → 6CO₂ (aq) + 6H₂O (l) + energy
3. Decomposition of vegetable matter into compost
Decomposition Reaction
Definition
- Single reactant breaks down into simpler products
- General form: AB → A + B
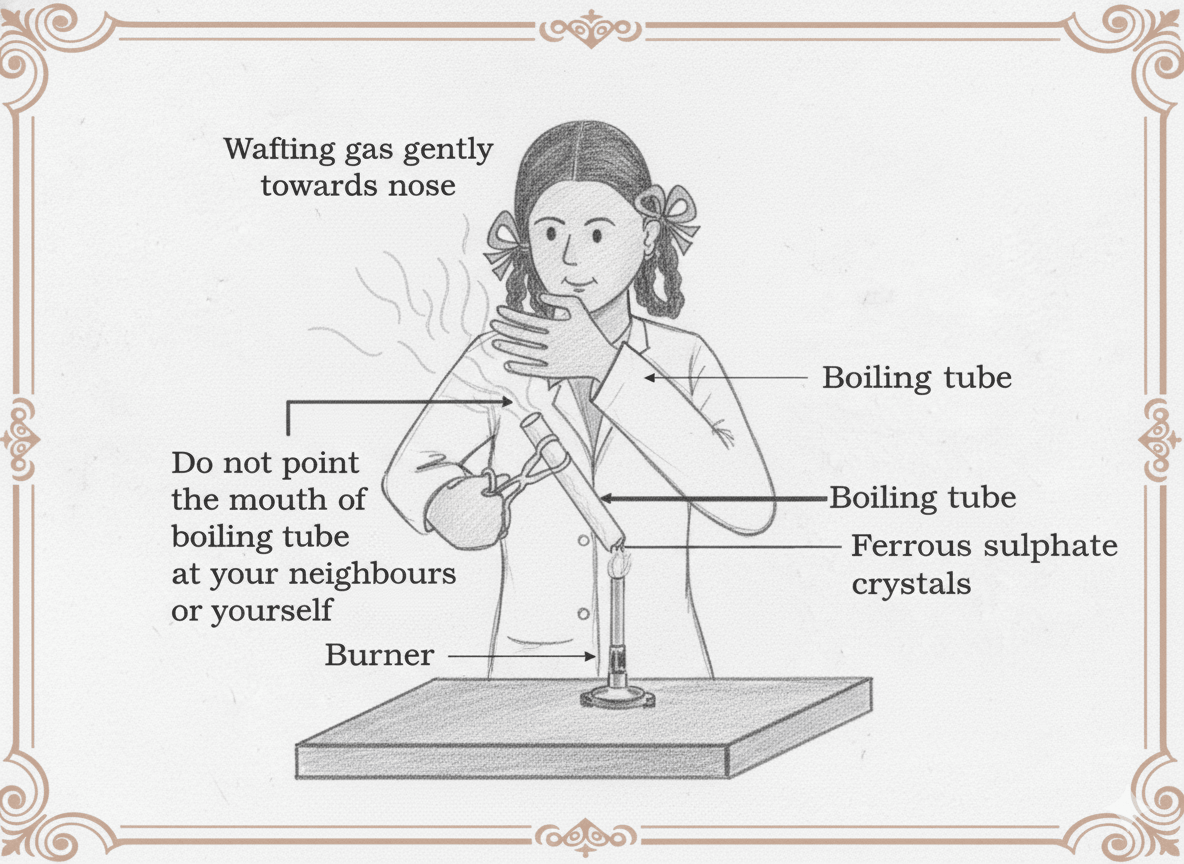
Thermal Decomposition
By heating
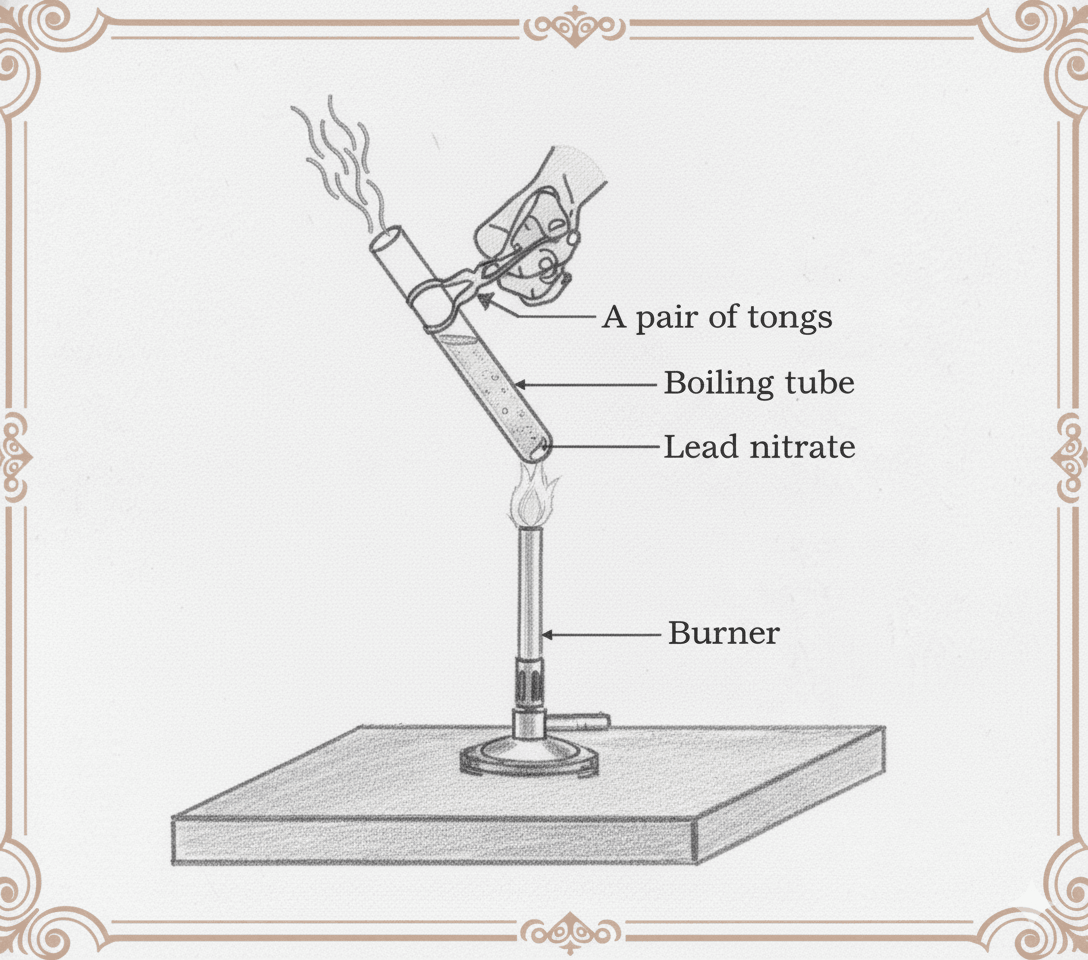
1. 2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g)
FeSO₄ = Ferrous sulphate
Fe₂O₃ = Ferric oxide
2. CaCO₃(s) → CaO(s) + CO₂(g)
CaCO₃ = Limestone
CaO = Quick lime
Decomposition by Light
1. 2AgCl(s) → 2Ag(s) + Cl₂(g)
The white color of AgCl changes to grey
2. 2AgBr(s) → 2Ag(s) + Br₂(g)
The above two reactions are used in black and white photography

Endothermic Reaction
- Energy is absorbed (heat, light, electricity)
- Decomposition reactions require energy
Displacement Reaction

Definition
A more reactive element displaces
less reactive element from its compound
Examples:
- Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
| Fe | Iron |
| CuSO₄ | copper sulphate |
| FeSO₄ | Iron sulphate |
| Cu | Copper |
- Here Iron has displaced copper from from copper sulphate solution.
- The iron becomes Brown and the blue color of CuSO₄ fades.
2. Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
3. Pb(s) + CuCl₂(aq) → PbCl₂(aq) + Cu(s)
- Zinc and lead are more reactive than copper
- They displace copper from its compounds
Double Displacement Reaction

Definition:
reactions in which there is an
exchange of ions between the reactants are called double displacement
reactions.
- Exchange of ions between reactants
- Often produces a precipitate (insoluble substance)
| Na₂SO₄ | Sodium sulphate |
| BaCl₂ | Barium chloride |
| BaSO₄ | Barium sulphate |
| 2NaCl | Sodium chloride |
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
Precipitation Reaction
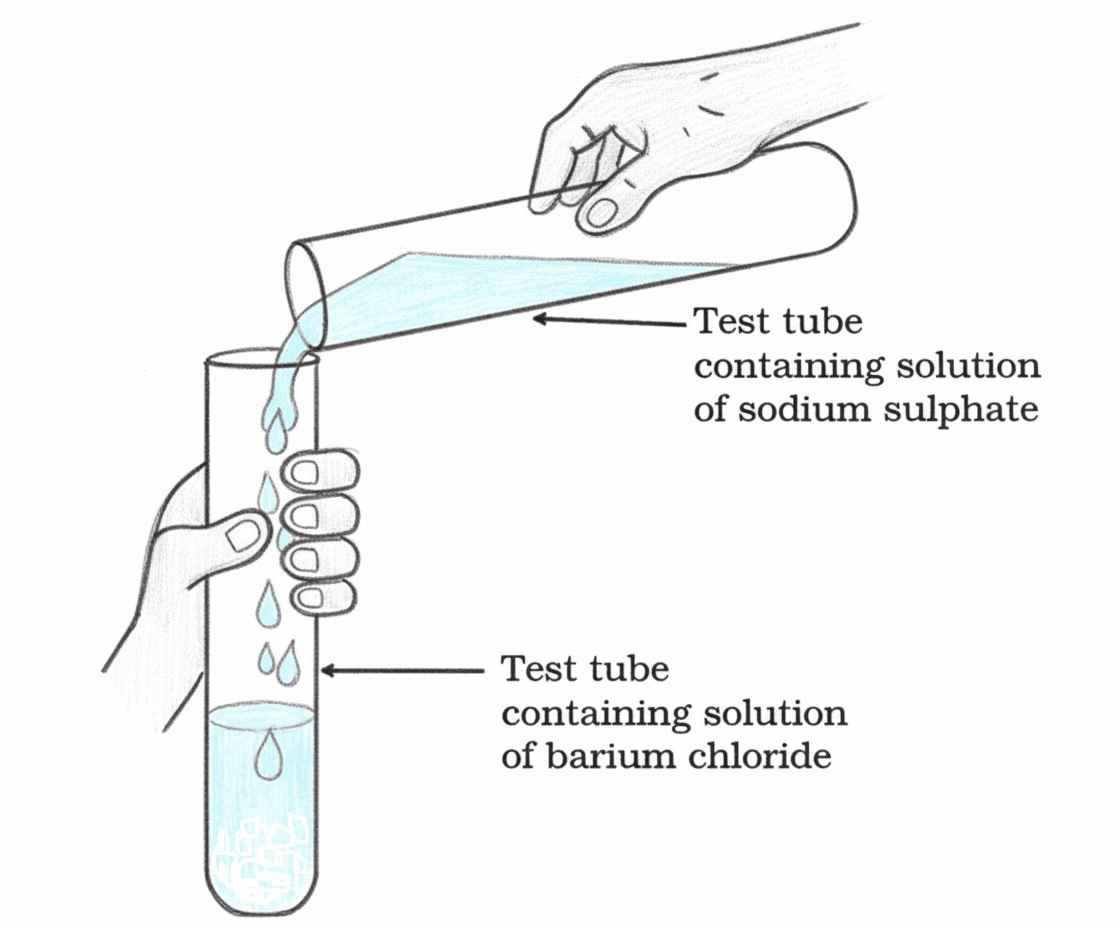
Any reaction that produces a precipitate. Precipitate is an insoluble substance.
In the above reaction, a white Precipitate (ppt.) of BaSO₄ is formed
- White precipitate of BaSO₄ formed
- Caused by reaction between SO₄²⁻ and Ba²⁺ ions
- Sodium chloride remains in solution
Oxidation and Reduction
Definitions of oxidation and reduction
Oxidation:
- Substance gains oxygen
- Substance loses hydrogen
Reduction:
- Substance loses oxygen
- Substance gains hydrogen
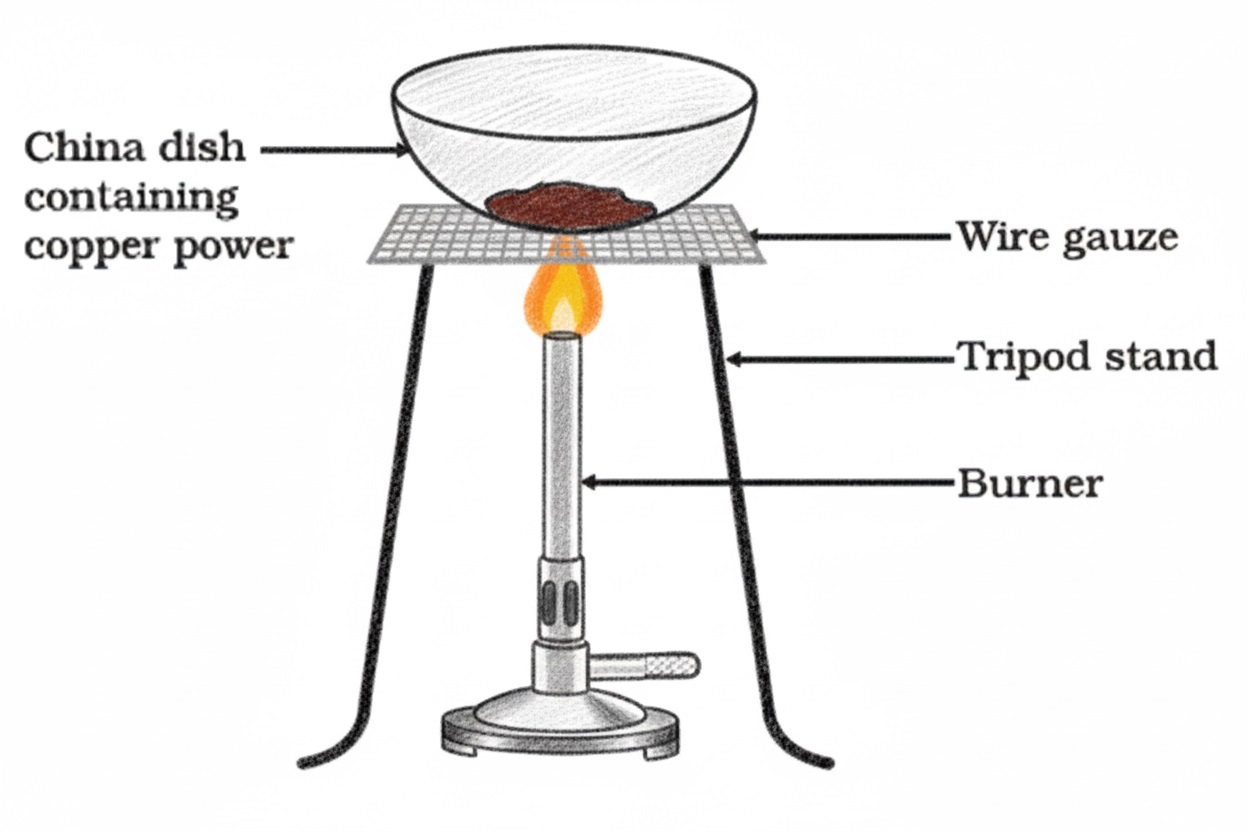
Redox Reactions
- One reactant gets oxidised while the other gets reduced
- Also called oxidation-reduction reactions
Examples of redox reactions:
- 2Cu + O₂ → 2CuO
Copper gains oxygen → oxidized - CuO + H₂ → Cu + H₂O
Copper oxide loses oxygen → reduced
Hydrogen gains oxygen → oxidized
- ZnO + C → Zn + CO
Carbon gains oxygen → oxidized
ZnO loses oxygen → reduced - MnO₂ + 4HCl → MnCl₂ + 2H₂O + Cl₂
HCl loses hydrogen → oxidized
MnO₂ loses oxygen → reduced
Corrosion
Definition
- Metal attacked by moisture, acids, etc.
- Process is called corrosion
Examples of corrosion :
- Silver: Black coating
- Iron: Shiny when new, gets reddish-brown coating (rusting)
- Copper: Green coating
Impact Of Corrosion
- Damages car bodies, bridges, ships, iron objects
- Serious problem for iron
- Enormous money spent on replacements
Rancidity
Definition
- Oxidation of fats and oils
- Becomes rancid – smell and taste change
Prevention
- Add antioxidants (prevent oxidation)
- Keep in airtight containers
- Flush with nitrogen gas (e.g., chip bags)