Four things you must do to get maximum out of the “Chemical Reactions And Equations 2 Marks Questions Class 10 “
First, read the Chapter 1 chemical reactions and equations from NCERT science textbook
Second, prepare our Short Notes on chemical reactions and equations
Third, revise with our chemical reactions and equations flow charts
And finally, solve the MCQs Quiz on chemical reactions and equations prepared by us.
Please note that the answers to the questions are exhaustive. By exhaustive, we mean that the answers are more explanatory and has additional information. This was done to make the answers understandable and easy to learn.
Q1. A magnesium ribbon is cleaned before burning in air. Why should it be cleaned and what will happen if it is not cleaned?
Magnesium ribbon is cleaned to remove the thin layer of magnesium oxide formed on its surface due to exposure to air.
If not cleaned, this oxide layer will prevent magnesium from burning properly in air and the reaction may not start easily.
Q2. A small piece of calcium oxide is added to water in a beaker. The beaker becomes warm. What type of reaction is this? Write its chemical equation.
This is an exothermic combination reaction. Heat is released when calcium oxide reacts vigorously with water to produce calcium hydroxide.
The balanced chemical equation for the reaction is:
CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat
Q3. A student took 2 g of ferrous sulphate crystals in a boiling tube and heated it. The colour of the crystals changed from green to brown and a gas with a choking smell was released. Name the type of reaction and write its balanced chemical equation.
This is a thermal decomposition reaction, where a single compound breaks down into two or more simpler substances on heating.
The green ferrous sulphate decomposes to form brown ferric oxide and releases pungent-smelling gases—sulphur dioxide (SO₂) and sulphur trioxide (SO₃).
The balanced chemical equation is:
2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g)
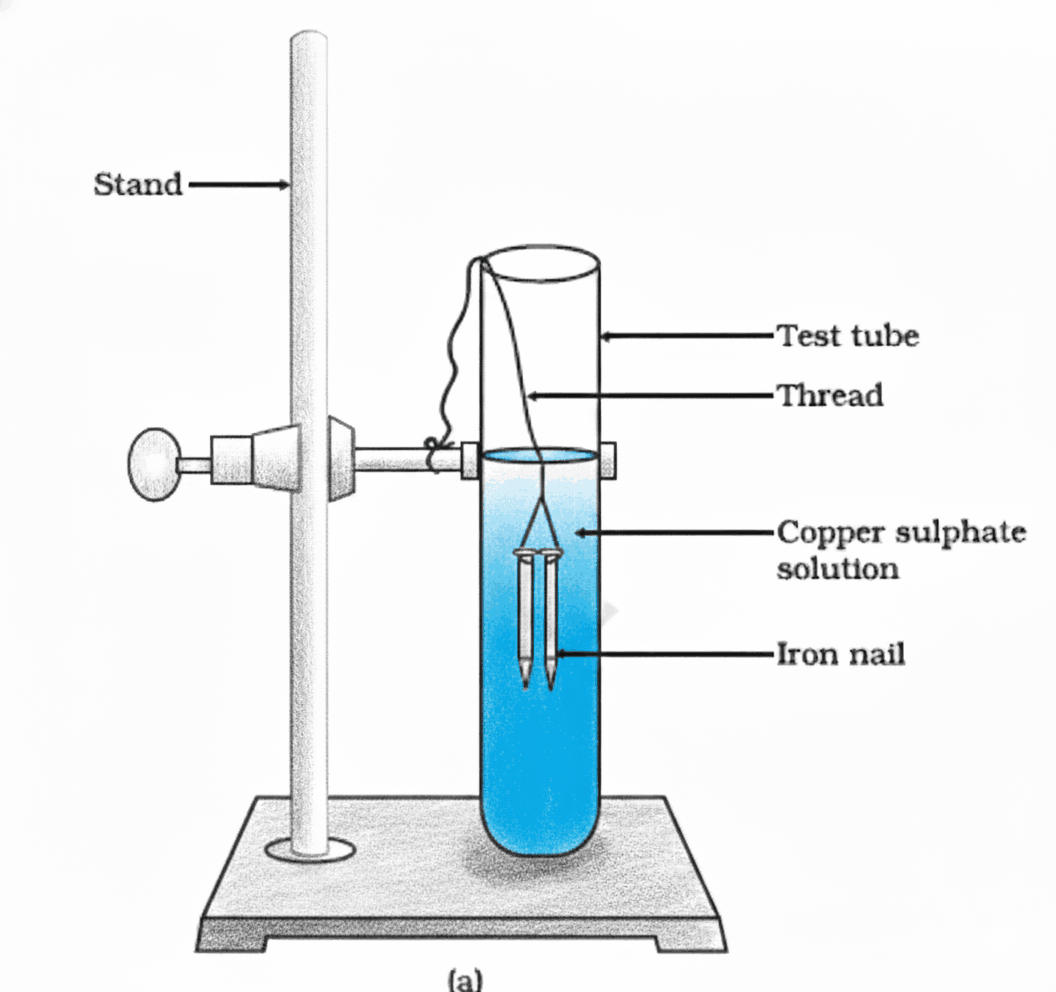
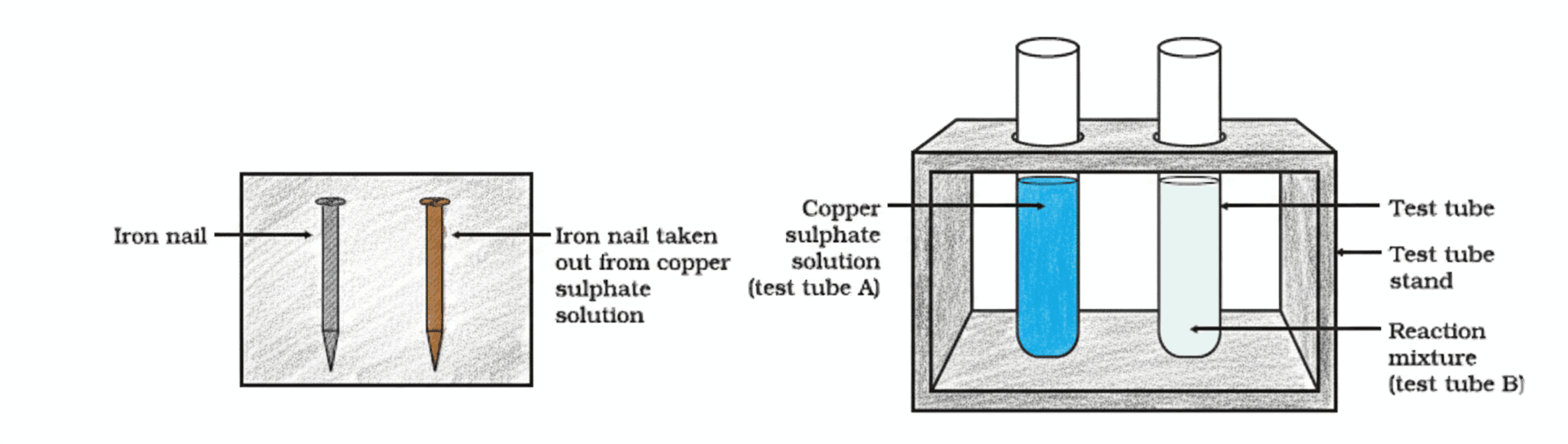
Q4. In an experiment, an iron nail was dipped in copper sulphate solution. After some time, the blue colour of the solution faded and a brown deposit formed on the nail. Explain the observation.
The observation occurs because iron is more reactive than copper. As a result, iron displaces copper from the copper sulphate solution in a single displacement reaction.
During the reaction:
- The blue colour of the solution fades because Cu²⁺ ions are removed from the solution as solid copper.
- A brown deposit of metallic copper forms on the surface of the iron nail.
- Iron goes into solution as Fe²⁺ ions, forming iron(II) sulphate.
The balanced chemical equation is:
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
Q5. A student exposed white silver chloride to sunlight and noticed a grey deposit forming. Name the reaction and explain the change.
This is a photochemical decomposition reaction—a type of decomposition driven by light energy.
When white silver chloride (AgCl) is exposed to sunlight, it breaks down into its constituent elements:
- Silver metal (Ag) is formed as a greyish deposit.
- Chlorine gas (Cl₂) is released (though it may dissipate or react further in air).
This reaction is why silver salts like AgCl are used in photographic films—they darken upon light exposure due to the formation of metallic silver.
The balanced chemical equation is:
2AgCl(s) → 2Ag(s) + Cl₂(g)
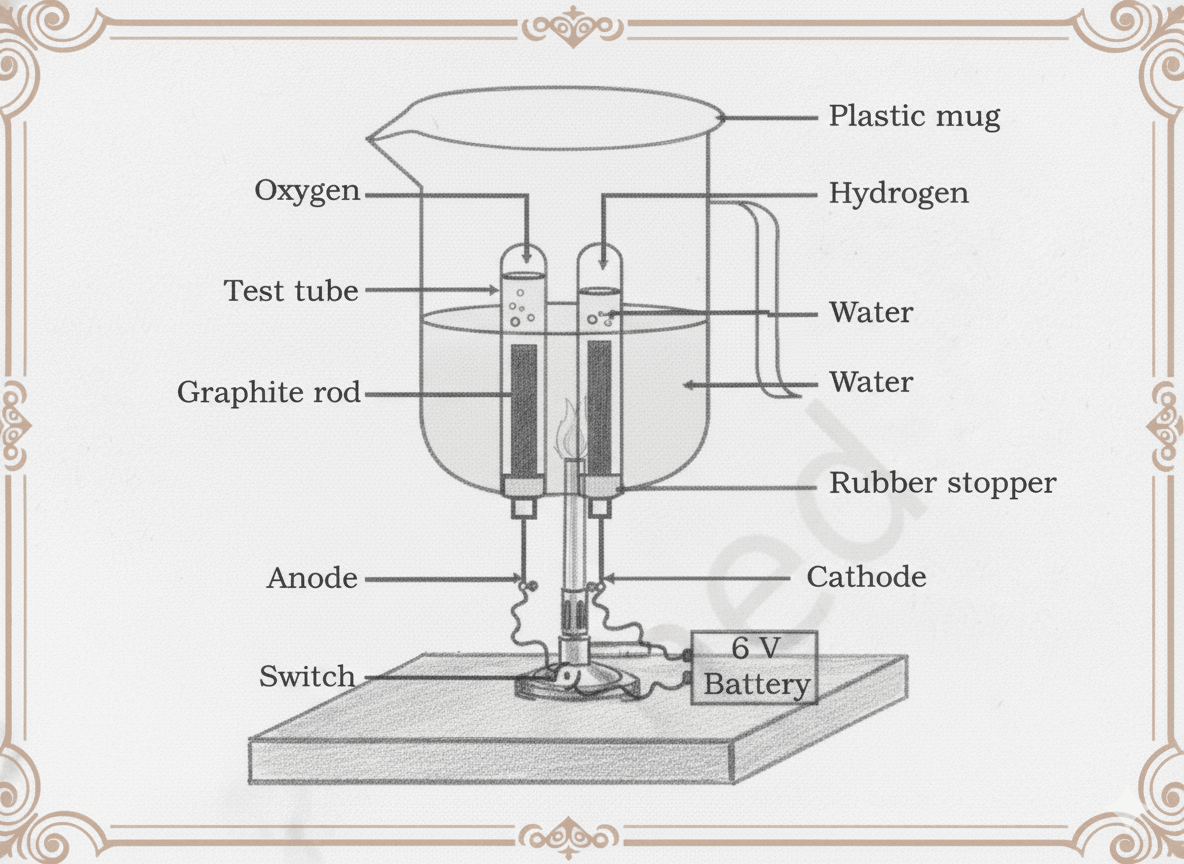
Q6. During electrolysis of water, the gas collected at one electrode is double the amount collected at the other. Identify the gases and explain why.
The two gases produced are hydrogen (H₂) and oxygen (O₂).
The volume of hydrogen gas is twice that of oxygen gas because a water molecule (H₂O) contains two hydrogen atoms for every one oxygen atom. During electrolysis, water decomposes in a fixed molar ratio, which directly translates to a 2:1 volume ratio of gases (as per Avogadro’s law).
The balanced chemical equation for the reaction is:
2H₂O(l) → 2H₂(g) + O₂(g)
Thus, for every 2 molecules of hydrogen gas produced, only 1 molecule of oxygen gas is formed—resulting in double the volume of hydrogen at the cathode compared to oxygen at the anode.
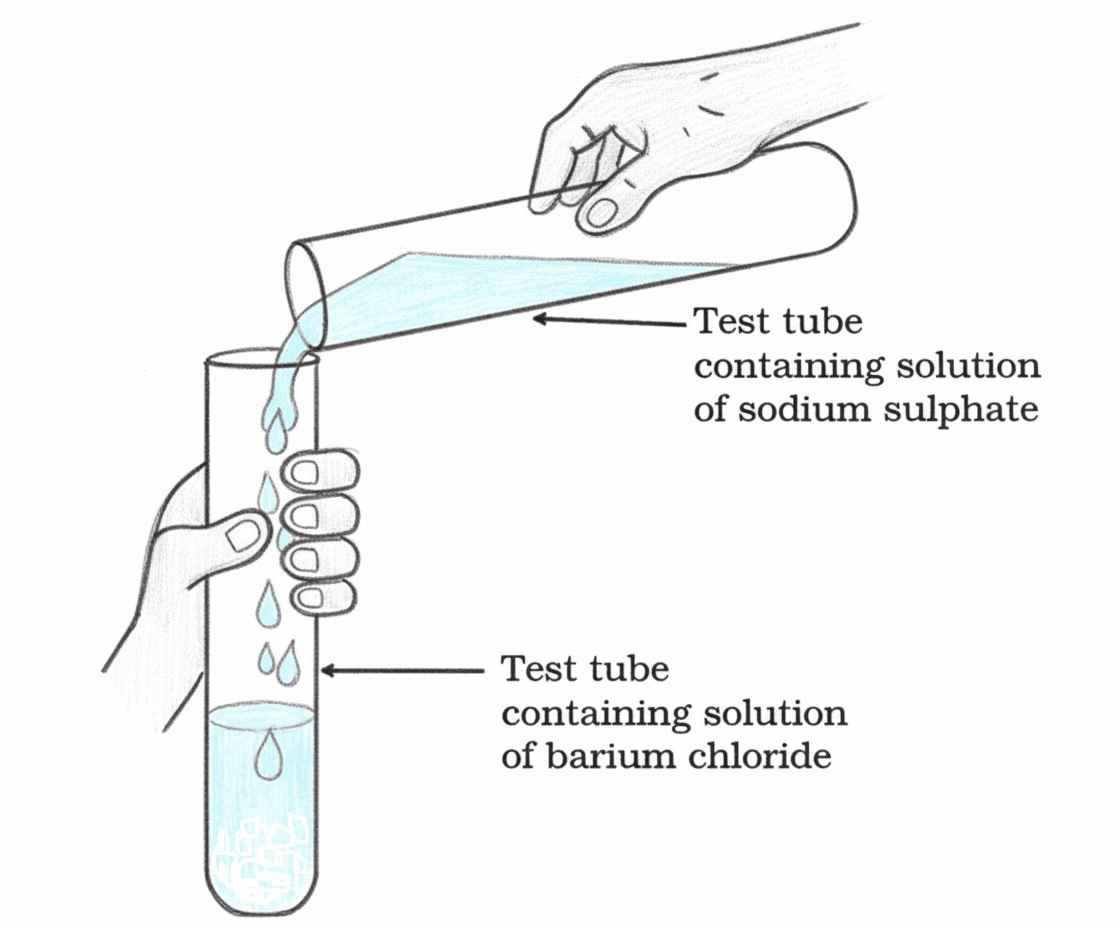
Q7. A student mixed sodium sulphate solution with barium chloride solution and observed a white precipitate. Identify the product and type of reaction.
The white precipitate formed is barium sulphate (BaSO₄).
This is a double displacement reaction, specifically classified as a precipitation reaction because an insoluble solid (precipitate) is produced when two aqueous solutions are mixed.
During the reaction, the Ba²⁺ ions from barium chloride combine with SO₄²⁻ ions from sodium sulphate to form insoluble BaSO₄, while Na⁺ and Cl⁻ remain in solution as sodium chloride.
The balanced chemical equation is:
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
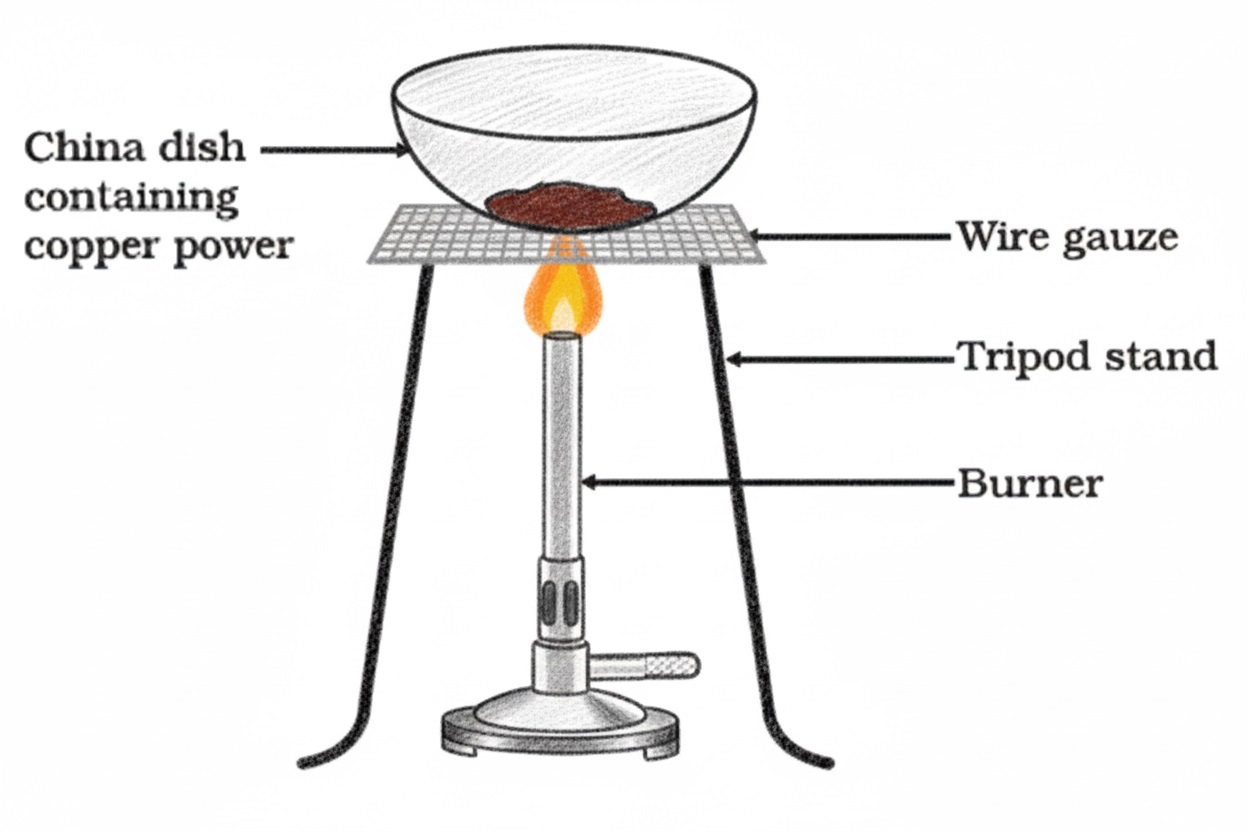
Q8. When copper powder is heated in air, it turns black. The black compound turns brown when hydrogen gas is passed over it. Name the two reactions and the substances involved.
Two sequential reactions occur:
1. Oxidation: When copper powder is heated in air, it reacts with oxygen to form black copper(II) oxide (CuO).
Chemical equation: 2Cu(s) + O₂(g) → 2CuO(s)
2. Reduction: When hydrogen gas is passed over the hot black CuO, it reduces back to brown metallic copper, and water vapour is formed.
Chemical equation: CuO(s) + H₂(g) → Cu(s) + H₂O(g)
Thus, copper is first oxidised to CuO and then reduced back to elemental copper.
Q9. Food containing fats and oils is stored in airtight containers or nitrogen-flushed packets. Why is this done?
This is done to prevent rancidity—a process in which fats and oils undergo oxidation when exposed to oxygen in the air.
Oxidation leads to unpleasant odours, off-flavours, and a loss of nutritional quality. By using airtight containers or nitrogen-flushed packaging, oxygen is excluded from the environment around the food.
Since nitrogen is an inert gas, it displaces oxygen and creates a protective atmosphere that slows down or prevents oxidative spoilage, thereby preserving the freshness, taste, and safety of the food for a longer time.
Q10. An iron gate left in the open for a few months develops a brown flaky coating. Name the process and write the chemical reaction.
The process is called corrosion, specifically known as rusting when it occurs in iron.
Rusting requires both oxygen and moisture (water). Initially, iron reacts with oxygen and water to form hydrated iron(III) hydroxide:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s)
This compound is unstable and gradually dehydrates to form hydrated iron(III) oxide, commonly written as Fe₂O₃·xH₂O, which appears as the familiar reddish-brown, flaky rust.
Rust does not adhere well to the metal surface, exposing fresh iron to further corrosion—making the process self-accelerating if left unchecked.
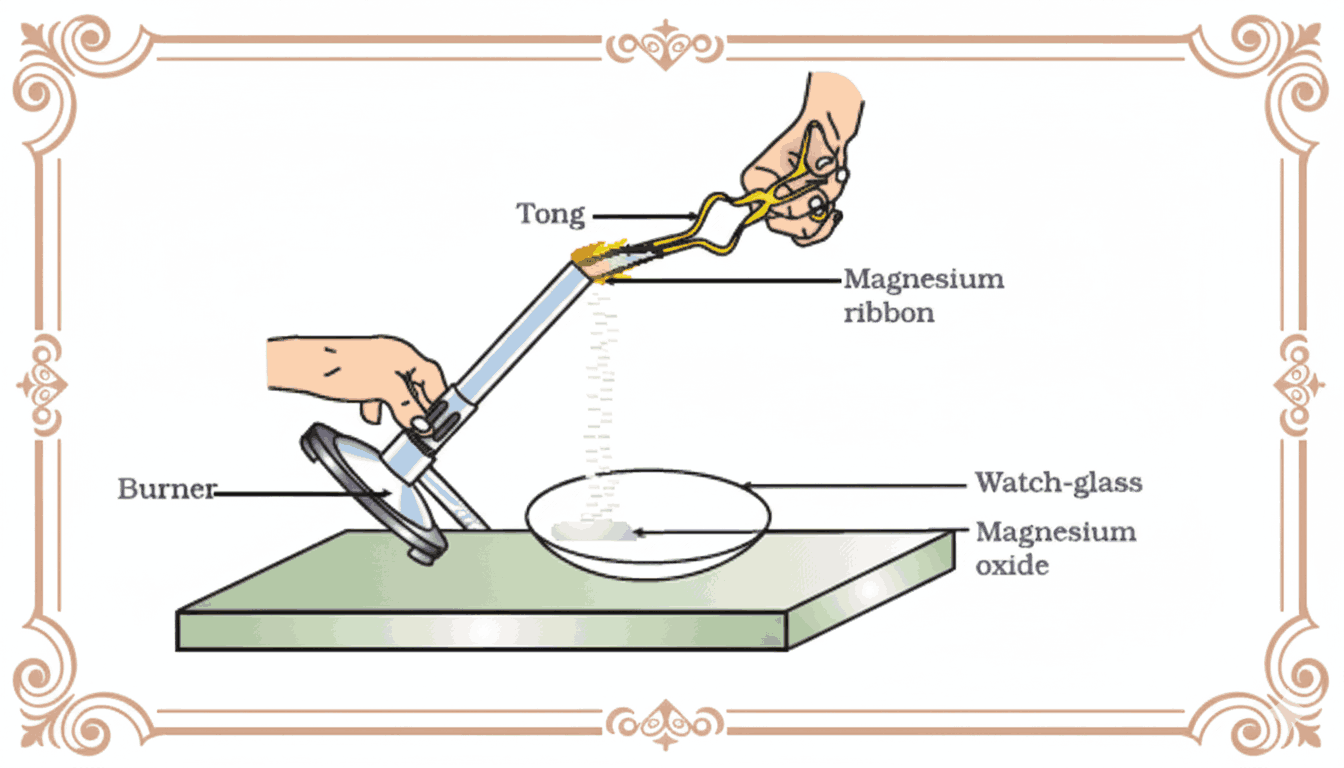
Q11. In the activity shown in Figure (burning of magnesium ribbon), a white ash collects in the watch glass. (a) Name the white ash formed. (b) Write the balanced chemical equation for the reaction.

(a) The white ash formed is magnesium oxide (MgO).
(b) The balanced chemical equation is:
2Mg(s) + O₂(g) → 2MgO(s)
This is a combination reaction, where magnesium combines with oxygen in the air to form magnesium oxide, releasing intense heat and bright white light.
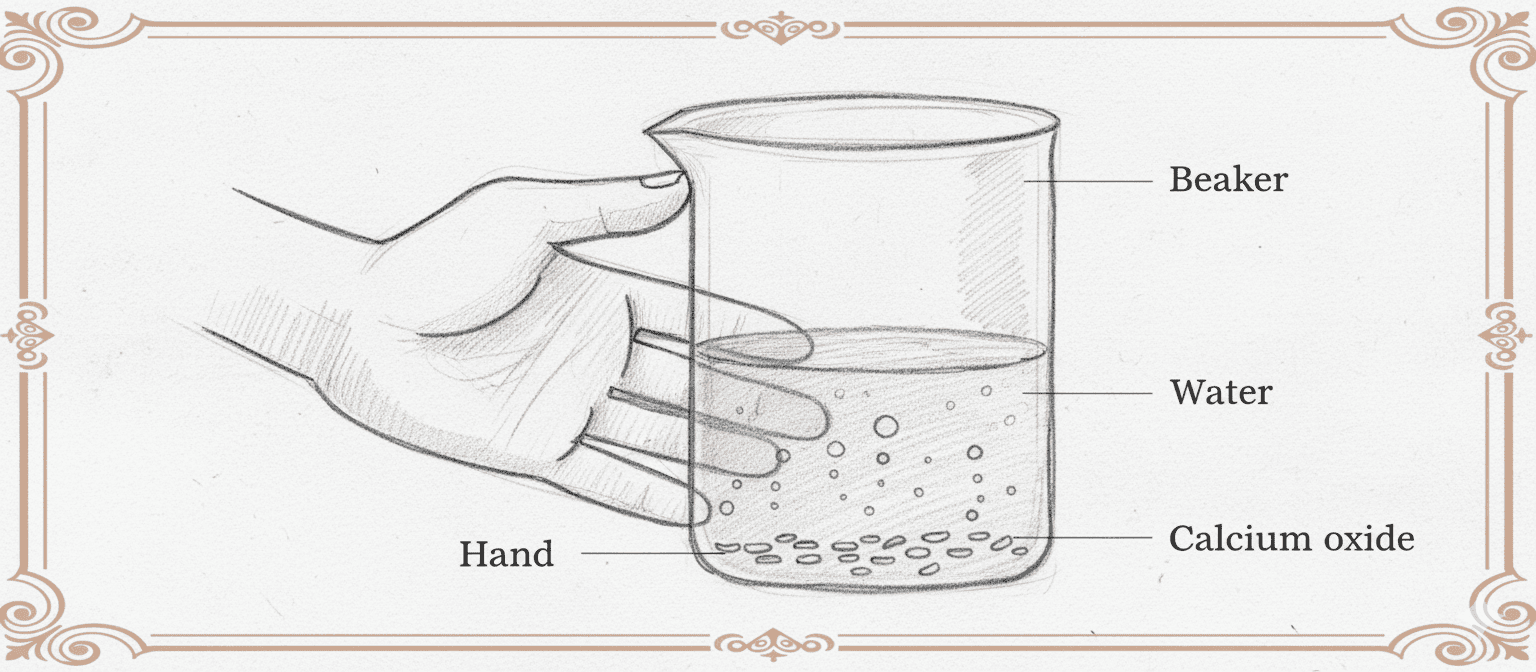
Q12. In Fig. calcium oxide reacts vigorously with water. (a) What do you observe when water is added to calcium oxide? (b) Name the type of reaction and write its chemical equation.

(a) When water is added to calcium oxide, you observe:
- A hissing sound due to rapid heat release.
- The mixture becomes very hot — sometimes even steaming.
- Formation of a white suspension called slaked lime (calcium hydroxide).
(b) This is an exothermic combination reaction.
The balanced chemical equation is:
CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat
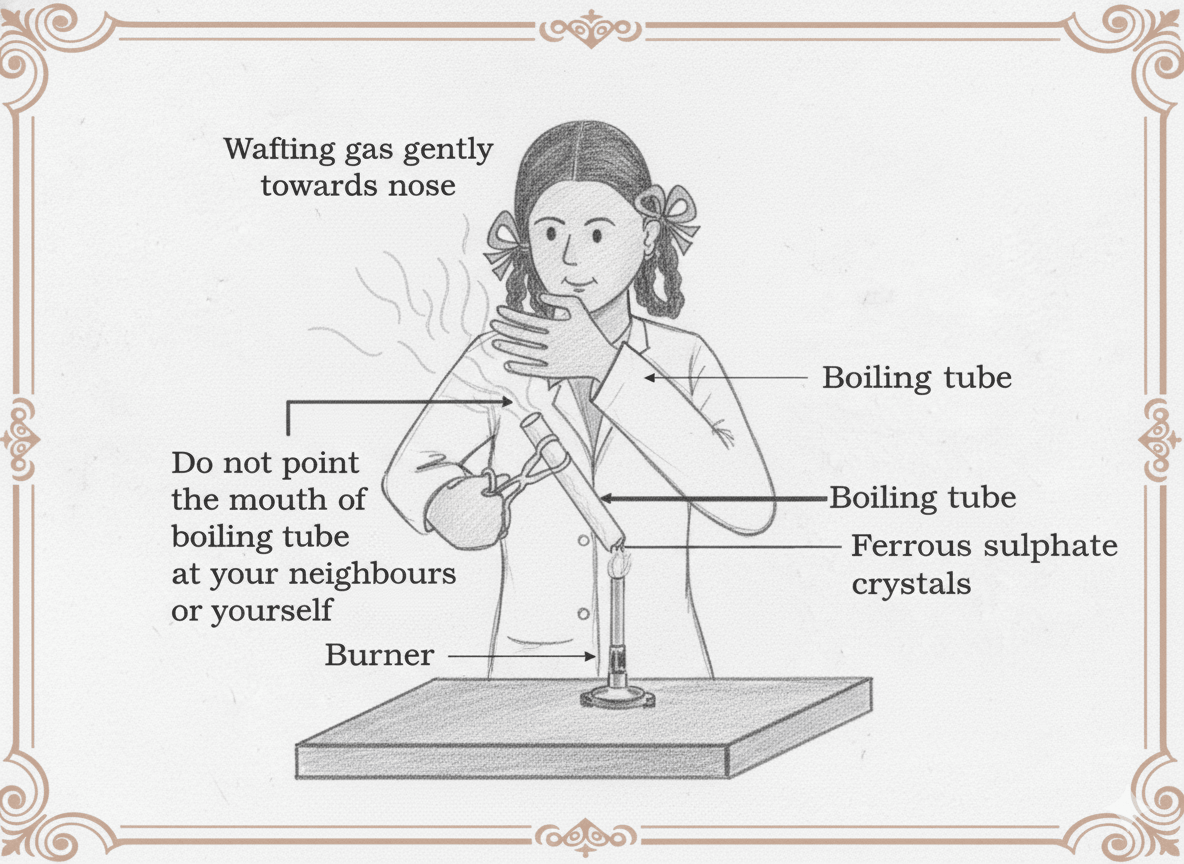
Q13. In Fig. Ferrous sulphate crystals are heated. (a) What change in colour is observed? (b) Write the balanced chemical equation for this reaction.

(a) The green colour of ferrous sulphate crystals changes to brown due to the formation of ferric oxide (Fe₂O₃).
A pungent-smelling gas (SO₂ and SO₃) is also released during heating.
(b) This is a thermal decomposition reaction. The balanced chemical equation is:
2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g)
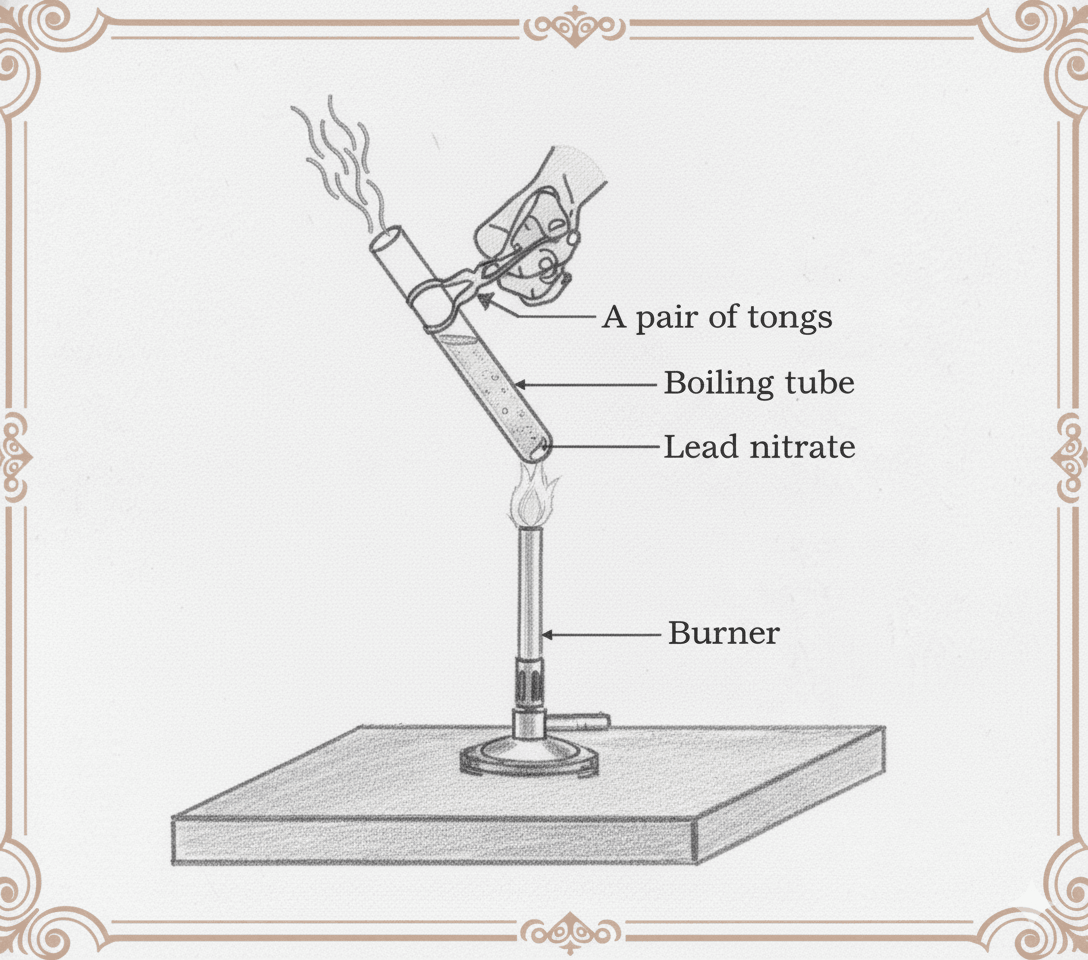
Q14. When lead nitrate is heated in a boiling tube, brown fumes are evolved. (a) Name the brown gas formed. (b) Write the balanced equation for the reaction.

(a) The brown fumes evolved are of nitrogen dioxide (NO₂) gas.
(b) This is a thermal decomposition reaction. The balanced chemical equation is:
2Pb(NO₃)₂(s) → 2PbO(s) + 4NO₂(g) + O₂(g)
During the reaction, solid lead nitrate breaks down on heating to form yellow lead(II) oxide (PbO), reddish-brown NO₂ gas, and colourless oxygen gas.
Q15. In Figure, water is decomposed by passing electric current through it. (a) Name the gases collected at the two electrodes. (b) Why is the volume of one gas double that of the other?

(a) During electrolysis of water:
- Hydrogen gas (H₂) is collected at the cathode (negative electrode).
- Oxygen gas (O₂) is collected at the anode (positive electrode).
(b) The volume of hydrogen gas is twice that of oxygen because a water molecule (H₂O) contains two hydrogen atoms for every one oxygen atom. This 2:1 atomic ratio translates directly into a 2:1 volume ratio of gases, as per Avogadro’s law.
The balanced chemical equation is:
2H₂O(l) → 2H₂(g) + O₂(g)
Q16. White silver chloride kept in sunlight turns grey after some time. (a) What causes the colour change? (b) Write the balanced chemical equation.
(a) The colour change from white to grey is caused by the photochemical decomposition of silver chloride when exposed to sunlight. The compound breaks down into metallic silver, which appears grey, and chlorine gas.
This reaction is light-sensitive and forms the basis of traditional black-and-white photography.
(b) The balanced chemical equation is:
2AgCl(s) → 2Ag(s) + Cl₂(g)
Q17. In the setup shown in Figure, iron nails are dipped in copper sulphate solution. (a) What colour change is observed in the solution? (b) Write the chemical equation and name the type of reaction.

(a) The following changes are observed:
- The blue colour of the copper sulphate solution fades (becomes greenish) as Cu²⁺ ions are removed.
- A brown coating of copper metal deposits on the iron nails.
(b) The reaction is a single displacement reaction (also called a substitution reaction), where iron—being more reactive than copper—displaces it from its salt solution.
The balanced chemical equation is:
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
Q18. In Figure, two clear solutions of sodium sulphate and barium chloride are mixed, and a white solid forms. (a) What is the white solid formed? (b) Write the balanced equation and name the type of reaction.

(a) The white solid formed is barium sulphate (BaSO₄), an insoluble salt.
(b) This is a double displacement reaction, also known as a precipitation reaction, because an insoluble solid (precipitate) forms when ions from two aqueous solutions exchange partners.
The balanced chemical equation is:
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
In this reaction, Ba²⁺ and SO₄²⁻ combine to form the precipitate, while Na⁺ and Cl⁻ remain in solution as spectator ions.
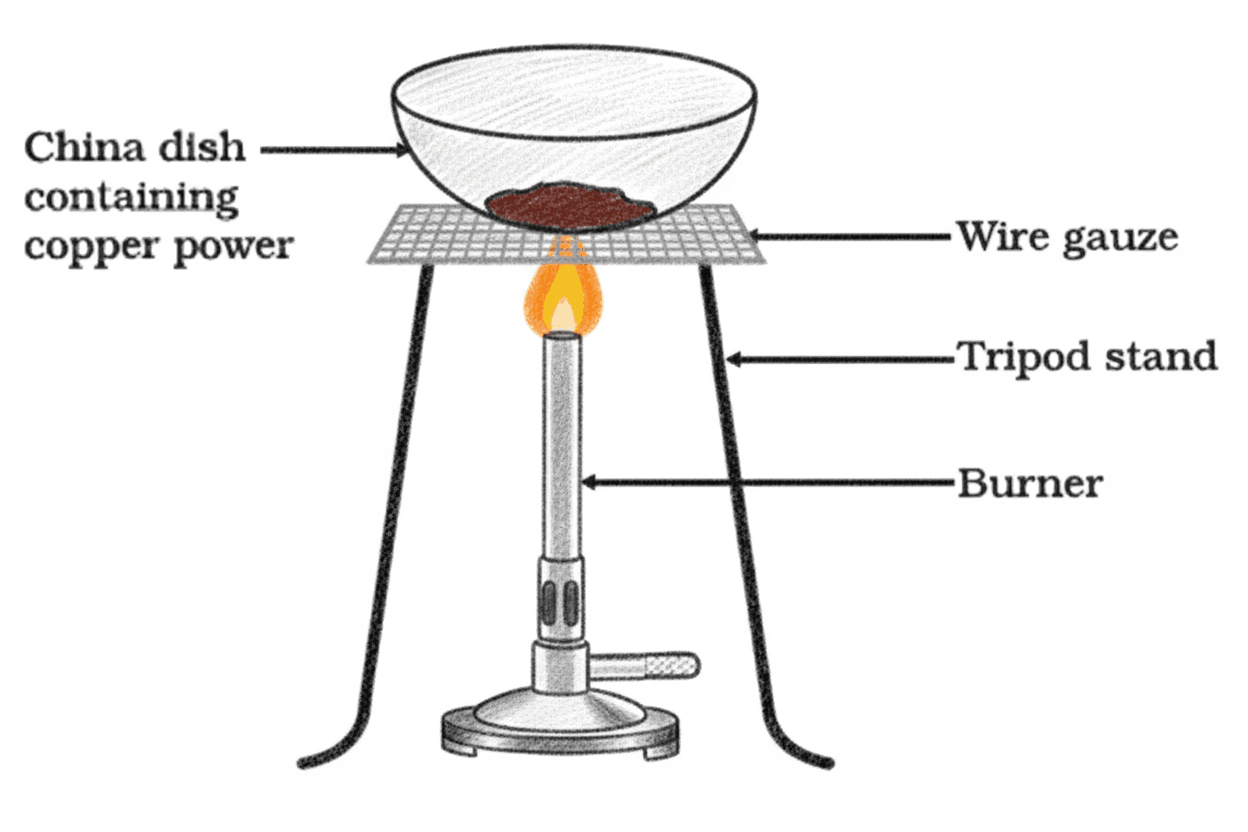
Q19. In Figure, copper powder is heated in a china dish. (a) What change is observed in the colour of copper? (b) Write the chemical equations for both oxidation and reduction processes involved.

(a) When copper powder is heated in air, it turns from its original reddish-brown colour to black due to the formation of copper(II) oxide. When hydrogen gas is passed over the hot black residue, it turns back to brown metallic copper.
(b) Two redox reactions occur:
- Oxidation: Copper reacts with oxygen to form copper(II) oxide.
- Reduction: Copper(II) oxide is reduced back to copper by hydrogen gas.
2Cu(s) + O₂(g) → 2CuO(s)
CuO(s) + H₂(g) → Cu(s) + H₂O(g)
This demonstrates a classic redox reaction — where one substance is oxidized and another is reduced simultaneously.
Q20. During whitewashing, a freshly coated wall becomes shiny after two to three days. (a) Name the two compounds responsible for this reaction. (b) Write the balanced equation for the process.
(a) The two compounds involved are:
- Calcium hydroxide — Ca(OH)₂ (applied as slaked lime wash)
- Carbon dioxide — CO₂ (from the air)
They react slowly over 2–3 days to form a thin, hard, and shiny layer of calcium carbonate (CaCO₃), which gives the wall its characteristic lustre.
(b) The balanced chemical equation is:
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
This is a slow carbonation reaction and is essential for the durability and shine of traditional whitewash.
Q21. In the Figure, ferrous sulphate crystals are heated and gases are released. If you hold a glass rod dipped in lime water near the mouth of the tube, the lime water turns milky. (a) Which gas is responsible for this change? (b) Write the reaction involved.

(a) The gas responsible for turning lime water milky is sulphur dioxide (SO₂). Although both SO₂ and SO₃ are released during heating of ferrous sulphate, it is SO₂ that reacts with lime water to produce the milky appearance.
(b) The chemical reaction between sulphur dioxide and lime water (calcium hydroxide) is:
SO₂(g) + Ca(OH)₂(aq) → CaSO₃(s) + H₂O(l)
The white precipitate formed is calcium sulphite (CaSO₃), which causes the milky appearance. This test is commonly used to detect the presence of SO₂ gas.
Q22. In the setup shown in Figure, heating of lead nitrate produces brown fumes. A student tested the fumes with moist blue litmus paper. It turned red. (a) Name the gas responsible for this observation. (b) Why does it turn the litmus red?

(a) The gas responsible is nitrogen dioxide (NO₂), which appears as reddish-brown fumes during the thermal decomposition of lead nitrate.
(b) NO₂ turns moist blue litmus paper red because it dissolves in water (from the moist litmus) to form nitric acid (HNO₃) and nitrous acid (HNO₂), both of which are acidic:
3NO₂(g) + H₂O(l) → 2HNO₃(aq) + NO(g)
The formation of acid lowers the pH, causing the blue litmus to turn red — confirming the acidic nature of the gas.
Q23. In Figure, a student performed electrolysis of water but found no gas bubbles forming. (a) What could be the reason? (b) How can this be corrected?

(a) The reason no gas bubbles are forming is that pure water is a very poor conductor of electricity. It lacks sufficient ions to carry the electric current needed for electrolysis.
(b) This can be corrected by adding a small amount of a strong electrolyte such as dilute sulphuric acid (H₂SO₄) or sodium hydroxide (NaOH). These substances dissociate into ions, making the solution conductive and allowing electrolysis to proceed, resulting in the evolution of hydrogen and oxygen gases at the electrodes.
Q24. When calcium carbonate is heated in a hard glass test tube, calcium oxide and carbon dioxide are formed. (a) What type of reaction is this? (b) How can you test that CO₂ gas is evolved?
(a) This is a thermal decomposition reaction, where a single compound (calcium carbonate) breaks down into two or more simpler substances (calcium oxide and carbon dioxide) upon heating.
(b) To test for carbon dioxide gas, pass the evolved gas through clear lime water (a solution of calcium hydroxide). If CO₂ is present, the lime water turns milky white due to the formation of insoluble calcium carbonate:
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
The overall decomposition reaction is:
CaCO₃(s) → CaO(s) + CO₂(g)
Q25. A student places two test tubes side by side — one with AgCl in sunlight and another kept in the dark. (a) What difference will the student observe after 15 minutes? (b) Give a reason for the difference.
(a) After 15 minutes, the student will observe that:
- The test tube exposed to sunlight turns grey.
- The test tube kept in the dark remains white.
(b) This difference occurs because silver chloride (AgCl) undergoes photochemical decomposition when exposed to sunlight. The light energy breaks it down into silver metal (grey) and chlorine gas:
2AgCl(s) → 2Ag(s) + Cl₂(g)
In the absence of light (dark), this reaction does not occur, so the compound remains unchanged as white AgCl.
Q26. In Figure, if copper sulphate solution is replaced with silver nitrate solution, will a similar reaction occur with iron nails? Justify your answer with a reason.

Yes, a reaction will occur — and it is actually more vigorous than with copper sulphate.
Contrary to the provided answer, iron is more reactive than silver (as per the reactivity series: Fe > H > Cu > Ag). Therefore, iron can and does displace silver from silver nitrate solution.
The correct observations would be:
- The colourless silver nitrate solution turns greenish due to formation of iron(II) nitrate.
- A shiny grey or black deposit of silver metal forms on the iron nail.
The balanced chemical equation is:
Fe(s) + 2AgNO₃(aq) → Fe(NO₃)₂(aq) + 2Ag(s)
Thus, a displacement reaction does occur — and is readily observable. The original answer appears to be incorrect based on standard reactivity principles.
Q27. In Figure, when sodium sulphate solution is mixed with barium chloride solution, a white precipitate forms immediately. (a) What will happen if the precipitate is filtered and dried? (b) State one use of such precipitation reactions in industry or medicine.

(a) The white precipitate is barium sulphate (BaSO₄). When filtered and dried, it remains as a fine, white, insoluble powder that is chemically stable and does not decompose under normal conditions.
(b) Precipitation reactions like this are widely used in:
- Medical diagnostics: Barium sulphate itself is used as a radiocontrast agent in X-ray imaging of the digestive tract (barium meal/swallow) because it is opaque to X-rays and non-toxic due to its insolubility.
- Water treatment: To remove harmful ions (e.g., lead, sulphate) by converting them into insoluble precipitates.
- Qualitative analysis: In laboratories to detect specific ions (e.g., SO₄²⁻ using Ba²⁺).
Q28. In Figure, copper powder turns black on heating. If the same dish is covered to prevent oxygen entry, what will be observed?

If the china dish is covered to prevent oxygen from entering, no colour change will occur. The copper powder will remain its original reddish-brown colour.
This is because the black substance formed during normal heating is copper(II) oxide (CuO), which results from the oxidation of copper by atmospheric oxygen:
2Cu(s) + O₂(g) → 2CuO(s)
In the absence of oxygen (due to the covered dish), this oxidation reaction cannot take place, so copper remains unreacted.
Q29. When dilute hydrochloric acid is added to zinc granules, bubbles appear on the surface. (a) Identify the gas evolved. (b) Write the balanced chemical equation.
(a) The gas evolved is hydrogen (H₂). This can be confirmed by the characteristic ‘pop’ sound when a burning splint is brought near the gas.
(b) The balanced chemical equation is:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
This is a single displacement reaction (also called a metal-acid reaction), where zinc, being more reactive than hydrogen, displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas.
Q30. Two test tubes contain lead nitrate solution. A student adds potassium iodide solution to one and potassium chloride to another. (a) In which test tube will a visible change occur? (b) What type of reaction is it?
(a) A visible change occurs only in the test tube where potassium iodide (KI) is added. A bright yellow precipitate of lead iodide (PbI₂) forms immediately.
No visible change occurs with potassium chloride (KCl), because lead chloride (PbCl₂) is sparingly soluble and may not form a prominent precipitate under dilute conditions — and even if it does, it is white and less striking than the vivid yellow PbI₂.
(b) This is a double displacement (precipitation) reaction, where the ions exchange partners to form an insoluble product.
The balanced chemical equation is:
Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) ↓ + 2KNO₃(aq)
The downward arrow (↓) indicates the formation of a precipitate.
Q31. After whitewashing, walls appear dull at first but become shiny after two to three days. Explain why this happens.
Initially, the wall is coated with a suspension of calcium hydroxide (slaked lime, Ca(OH)₂), which appears dull and matte when dry.
Over the next 2–3 days, it slowly reacts with carbon dioxide (CO₂) from the air in a process called carbonation:
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
The product, calcium carbonate (CaCO₃), is a hard, white, crystalline solid—chemically similar to marble or limestone. This thin, smooth layer reflects light uniformly, giving the wall a shiny, lustrous finish.
Thus, the transformation from dull to shiny is due to the formation of this marble-like calcium carbonate coating on the surface.
Q32. Why do we apply paint on iron articles like bridges, gates, or railings?
We apply paint on iron articles to prevent corrosion (rusting). Paint forms a protective barrier that blocks moisture (water) and oxygen in the air from coming into direct contact with the iron surface—both of which are essential for rusting to occur.
Without this coating, iron undergoes a redox reaction in the presence of air and water, forming hydrated iron(III) oxide, commonly known as rust:
4Fe(s) + 3O₂(g) + 6H₂O(l) → 4Fe(OH)₃(s) → Fe₂O₃·xH₂O(s) (rust)
Rust is flaky, porous, and non-protective, so it exposes fresh iron to further corrosion. Painting significantly extends the lifespan of iron structures by interrupting this destructive process.
Q33. Why are chips packets flushed with nitrogen gas before sealing?
Chips packets are flushed with nitrogen gas (N₂) before sealing to prevent rancidity of the oils and fats present in the chips.
Nitrogen is an inert gas—it does not react with food components. By displacing oxygen from the packet, it stops oxidation, which would otherwise cause the fats to break down and produce unpleasant odours, flavours, and potentially harmful compounds.
This process also helps:
- Preserve crispness and freshness,
- Extend shelf life,
- Prevent the packet from collapsing (nitrogen provides cushioning).
Thus, nitrogen flushing is a safe, effective method to maintain the quality, taste, and safety of packaged snacks.
Q34. During respiration, energy is released from glucose in the body. Name the type of chemical reaction and write the balanced equation.
This is an exothermic oxidation reaction—specifically, aerobic respiration.
Glucose (C₆H₁₂O₆) reacts with oxygen (O₂) in the cells to produce carbon dioxide, water, and a large amount of usable energy (in the form of ATP), along with heat.
The balanced chemical equation is:
C₆H₁₂O₆(aq) + 6O₂(g) → 6CO₂(g) + 6H₂O(l) + Energy
The energy released is essential for vital life processes such as muscle contraction, nerve impulse transmission, and maintaining body temperature—keeping us warm and active.
Q35. When moist air comes in contact with silver jewellery, it becomes black. Why does this happen?
Silver jewellery turns black due to a corrosion reaction with trace amounts of hydrogen sulphide (H₂S) present in the air (from sources like polluted air, rubber, eggs, or industrial emissions).
In the presence of moisture, silver reacts with H₂S to form a thin, black layer of silver sulphide (Ag₂S):
2Ag(s) + H₂S(g) → Ag₂S(s) + H₂(g)
This black coating of Ag₂S is not rust (which occurs in iron), but it is a form of tarnishing—a specific type of corrosion that affects silver. The process is accelerated by humidity, pollutants, and prolonged exposure to air.
Q36. A copper vessel, after long use, turns green. (a) What is the green layer composed of? (b) Write the equation of the reaction.
(a) The green layer is known as basic copper carbonate, with the chemical formula CuCO₃·Cu(OH)₂. It is also called patina.
(b) This layer forms due to the corrosion of copper when exposed over time to moist air containing carbon dioxide and oxygen. The overall reaction is:
2Cu(s) + H₂O(l) + CO₂(g) + O₂(g) → CuCO₃·Cu(OH)₂(s)
This green coating is adherent and protective—unlike rust on iron—and prevents further corrosion of the underlying copper metal. It is commonly seen on old copper roofs, statues (like the Statue of Liberty), and traditional cookware.
Q37. Why is respiration considered an exothermic reaction?
Respiration is considered an exothermic reaction because it involves the breakdown of glucose in the presence of oxygen, which releases energy in the form of heat and chemical energy (ATP).
The overall chemical equation is:
C₆H₁₂O₆(aq) + 6O₂(g) → 6CO₂(g) + 6H₂O(l) + Energy
Since energy is liberated (not absorbed) during this process, it is classified as exothermic. This released energy is essential for vital life processes such as:
- Muscle contraction
- Nerve impulse transmission
- Maintaining body temperature
- Cellular metabolism
Thus, respiration is a controlled, biological oxidation reaction that gives off energy—the hallmark of an exothermic process.
Q38. Quicklime (CaO) is added to moist soil during the construction process. The mixture becomes hot. (a) What kind of reaction is this? (b) Write the balanced equation.
(a) This is an exothermic combination reaction. When quicklime (calcium oxide) comes into contact with water, it combines vigorously to form slaked lime (calcium hydroxide), releasing a significant amount of heat in the process.
(b) The balanced chemical equation is:
CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat
The heat released makes the mixture noticeably hot—this property is utilized in construction to prepare lime mortar and to stabilize soil. The reaction is highly exothermic and must be handled with care.
Q39. Why is iron nail coated with zinc metal before use (a process called galvanisation)?
Iron nails are coated with zinc through a process called galvanisation to prevent rusting (corrosion).
Although zinc is more reactive than iron, it forms a thin, adherent, and insoluble layer of zinc oxide (ZnO) or zinc carbonate (ZnCO₃) when exposed to air. This layer acts as a protective barrier that prevents moisture and oxygen from reaching the underlying iron.
Even if the coating is scratched, zinc continues to protect iron by acting as a sacrificial anode—it oxidises preferentially, thereby sacrificing itself to save the iron.
Thus, galvanisation provides dual protection: barrier protection and electrochemical (sacrificial) protection—making it highly effective for outdoor and structural iron applications.

![MCQs Light Reflection And Refraction Class 10 [Quiz Format], Important Questions!](https://studyless.in/wp-content/uploads/2026/01/Short-2_11zon.jpg)


