Acids Bases And Salts Short Notes Class 10 is NCERT based for fast revison and retention. Every importnat point of the chapter 2 of chemistry for class 10 is included in the notes.
Acids Bases And Salts Short Notes Class 10 will work best if you had read the chapter 2 from your text book thoroughly. Beacuse this chapter also has many chemical reactions like the chapter 1
Introduction
Taste
- Sour taste → acids (e.g., lemon juice, vinegar)
- Bitter taste → bases (e.g., baking soda, soap)
Acidity Remedy
- For acidity after overeating → use a baking soda solution
- Reason: Baking soda is basic and neutralises excess stomach acid
Neutralization
- Acids and bases cancel each other’s effects
- This reaction is called neutralization
Testing Without Tasting
- Litmus paper:
- Acid turns blue litmus → red
- Base turns red litmus → blue
- Turmeric:
- Yellow in acid/neutral
- Turns reddish-brown in base (e.g., curry stain + soap)
- Returns to yellow when rinsed with water
- Synthetic indicators:
- Methyl orange and phenolphthalein also detect acids/bases
Understanding Chemical Properties of Acids and Bases
Common Indicators & Colour Changes
- Blue litmus solution: Turns red in acid
- Red litmus solution: Turns blue in base
- Phenolphthalein:
- Colourless in acid
- Pink in base
- Methyl orange:
- Red in acid
- Yellow in base
Olfactory Indicators
- Substances whose smell changes in acidic or basic conditions
- Examples: vanilla, onion, clove
- In basic medium:
- Smell of vanilla, onion, or clove fades or disappears
- In acidic medium:
- Their original smell remains
How Acids and Bases React with Metals
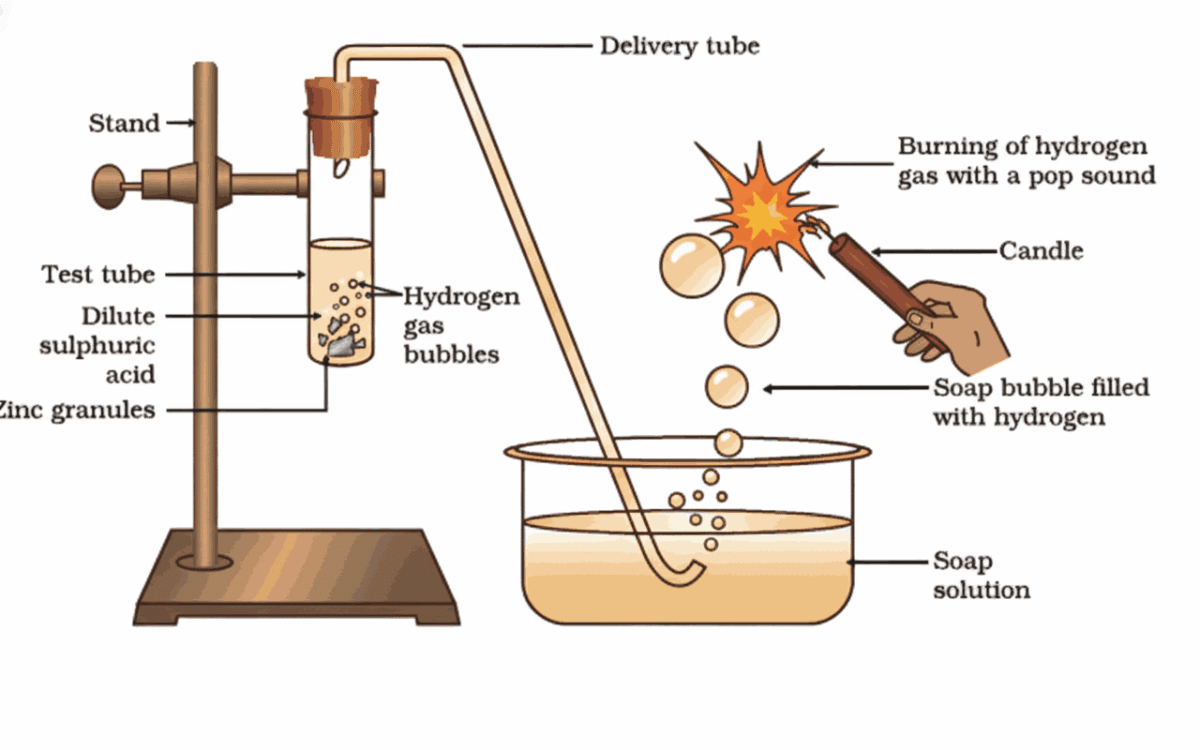
Reaction of Metals with Acids
- Acids react with certain metals to produce hydrogen gas and a salt
- General equation:
Acid + Metal → Salt + Hydrogen gas - Example:
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
Reaction of Metals with Bases
- Some metals (e.g., zinc, aluminium) also react with strong bases like NaOH
- They produce hydrogen gas and a complex salt
- Example:
2NaOH(aq) + Zn(s) → Na₂ZnO₂(s) + H₂(g)
(Sodium zincate + Hydrogen gas)
Important Points
- Not all metals react with acids or bases
- Only reactive metals (above hydrogen in the reactivity series) displace hydrogen from acids
- Metals like copper, silver, gold do not react with most acids or bases
Reaction of Metal Carbonates & Hydrogencarbonates with Acids
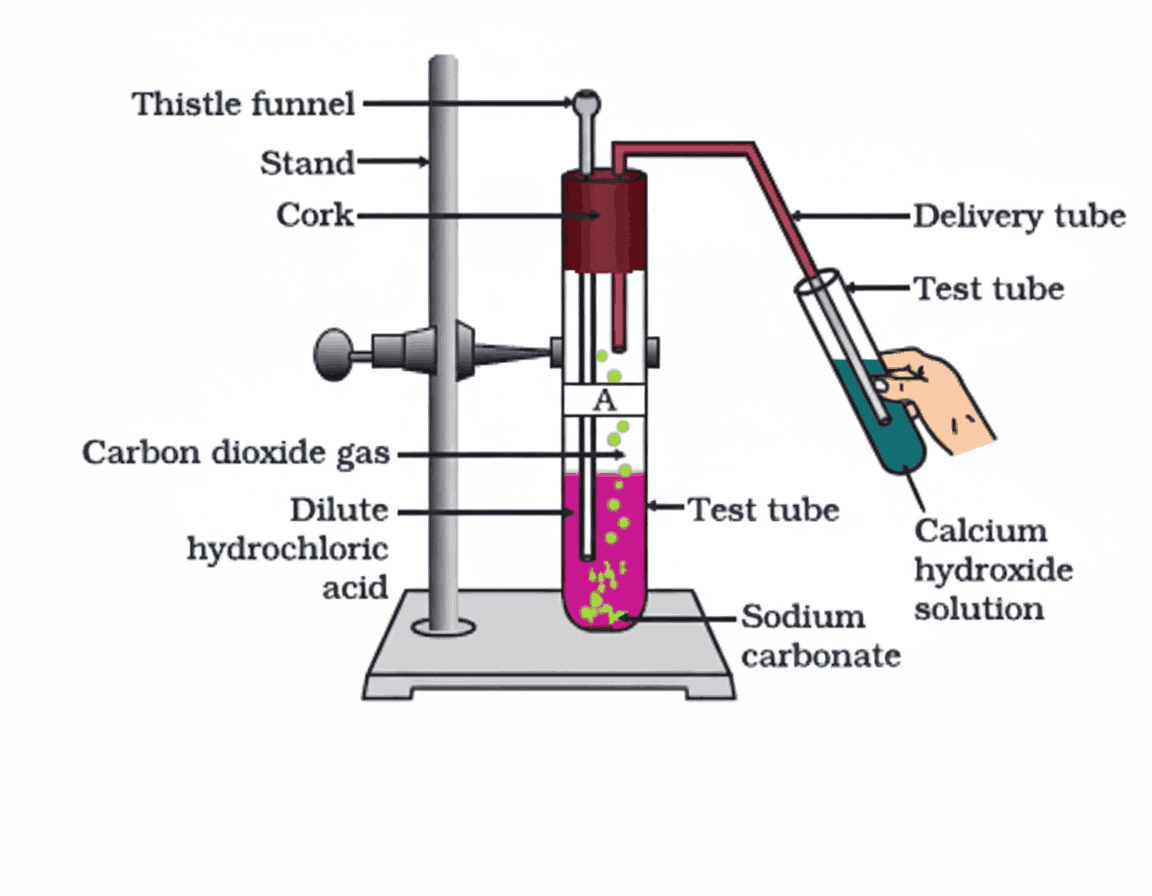
General Reaction
Metal carbonate / Metal hydrogencarbonate + Acid → Salt + Carbon dioxide + Water
Example Reactions
- Sodium carbonate:
Na₂CO₃(s) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g) - Sodium hydrogencarbonate (baking soda):
NaHCO₃(s) + HCl(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
Testing for Carbon Dioxide
- Pass CO₂ gas through lime water (Ca(OH)₂):
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
→ Lime water turns milky due to white CaCO₃ precipitate - With excess CO₂:
CaCO₃(s) + H₂O(l) + CO₂(g) → Ca(HCO₃)₂(aq)
→ Milky colour disappears as soluble calcium hydrogencarbonate forms
Common Carbonates in Daily Life
- Limestone, chalk, marble = natural forms of calcium carbonate (CaCO₃)
Key Takeaway
- All metal carbonates and hydrogencarbonates produce CO₂ gas when reacted with acids
- This reaction is used in fire extinguishers, baking, and acid neutralisation
How do Acids and Bases React with each other?
Neutralisation Reaction
- When an acid reacts with a base, they cancel each other’s effect
- Products: Salt + Water
- General equation:
Base + Acid → Salt + Water
Example
- Sodium hydroxide + Hydrochloric acid → Sodium chloride + Water
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
Key Features
- No gas is evolved
- The resulting solution is neutral (pH ≈ 7) if acid and base are used in equal strength
- Heat is often released → exothermic reaction
Everyday Uses
- Treating acidity (antacids neutralise stomach acid)
- Soil treatment (adjusting pH for crops)
- Industrial waste neutralisation before disposal
Reaction of Metallic Oxides with Acids
General Reaction
- Metal oxide + Acid → Salt + Water
- Metallic oxides behave like bases → called basic oxides
Example:
Copper(II) Oxide + Hydrochloric Acid
- Observation: Black copper oxide dissolves, forming a blue-green solution
- Product: Copper(II) chloride (CuCl₂) and water
- Balanced equation:
CuO(s) + 2HCl(aq) → CuCl₂(aq) + H₂O(l)
Why Are Metallic Oxides Basic?
- They neutralise acids just like bases do
- Produce salt and water—no gas evolved
- Confirm basic nature through neutralisation reactions
Key Takeaway
- Most metallic oxides are basic
- They react with acids in a neutralisation-like reaction to form salt and water
Reaction of Non-Metallic Oxides with Bases
General Behaviour
- Non-metallic oxides react with bases to form salt + water
- This is similar to an acid–base neutralisation → so non-metallic oxides are acidic in nature
Example:
Carbon Dioxide + Calcium Hydroxide
- CO₂ (non-metallic oxide) + Ca(OH)₂ (base) → CaCO₃ (salt) + H₂O
- Balanced equation:
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l) - Observation: Lime water turns milky due to white CaCO₃ precipitate
Why Are Non-Metallic Oxides Acidic?
- They neutralise bases just like acids do
- Often form acidic solutions in water (e.g., CO₂ + H₂O → H₂CO₃, carbonic acid)
Common Acidic Oxides
- CO₂, SO₂, NO₂, P₄O₁₀
Key Takeaway
- Non-metallic oxides = acidic oxides
- They react with bases in a neutralisation-like reaction, confirming their acidic character
What Do All Acids and Bases Have in Common?
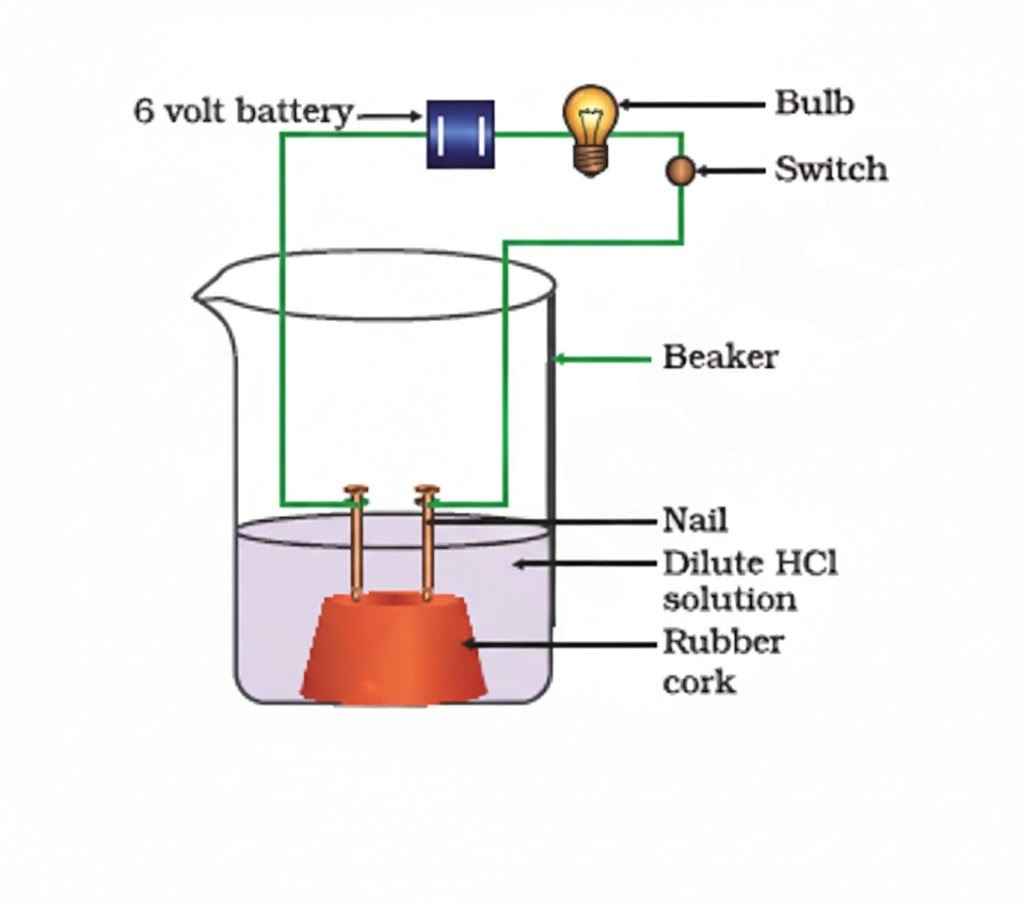
Common Feature of Acids
- All acids produce hydrogen ions (H⁺) in aqueous solution
- These H⁺ ions are responsible for acidic properties (e.g., sour taste, reactivity with metals, effect on indicators)
- Not all hydrogen-containing compounds are acids (e.g., glucose, alcohol don’t release H⁺ → not acidic)
Electrical Conductivity
- Acid solutions conduct electricity because
they contain mobile ions (H⁺ and anions like Cl⁻, NO₃⁻, etc.) - Glucose/alcohol solutions do not conduct → no ions formed
Common Feature of Bases (Alkalis)
- Bases like NaOH, Ca(OH)₂ produce hydroxide ions (OH⁻) in water
- OH⁻ ions are responsible for basic properties (bitter taste, slippery feel, effect on indicators)
- Base solutions also conduct electricity due to presence of ions (e.g., Na⁺ & OH⁻)
Key Takeaway
- Acids → release H⁺(aq) ions
- Bases → release OH⁻(aq) ions
- The presence of these ions explains similar chemical behaviour within acids and within bases
- Ion formation in water is essential for acidic or basic character
What Happens to an Acid or a Base in Water?
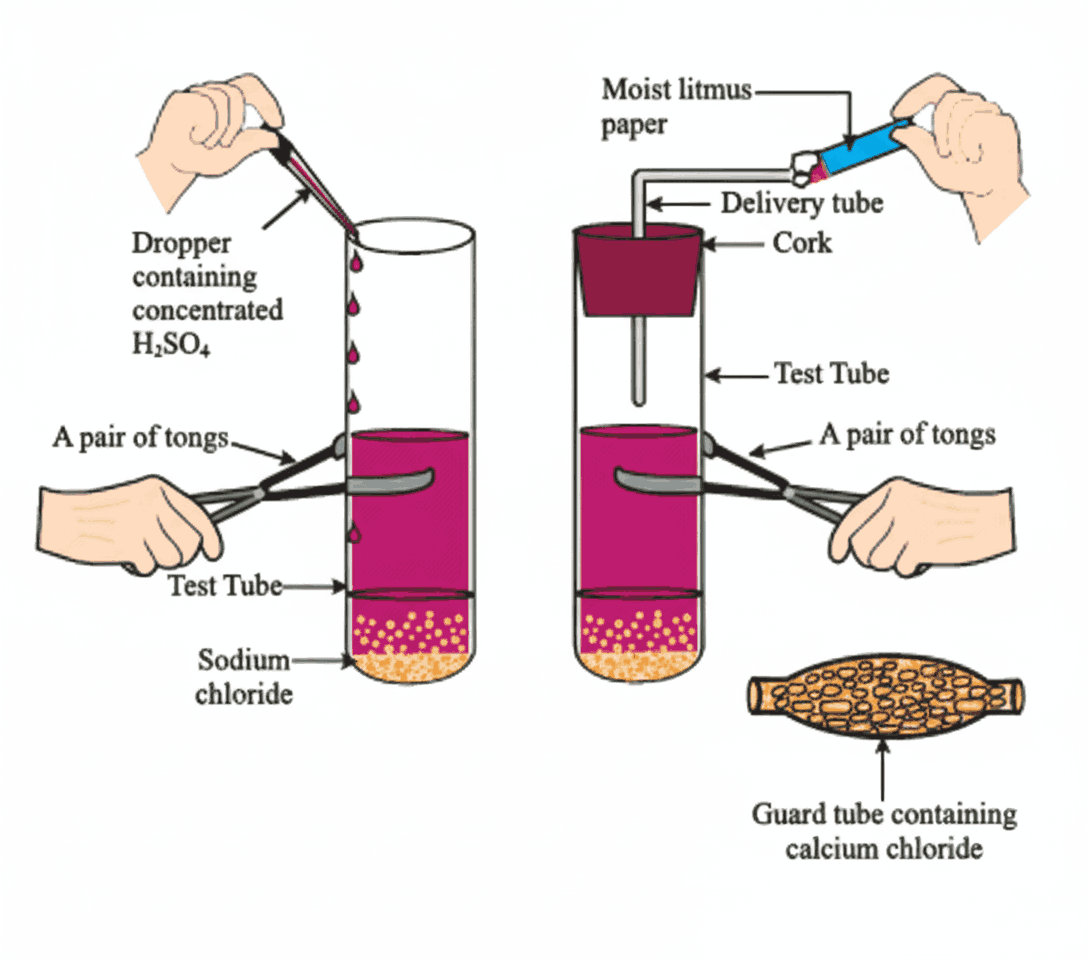
Acids in Water
- Acids release hydrogen ions (H⁺) only in the presence of water
- H⁺ ions instantly combine with water to form hydronium ions (H₃O⁺)
HCl + H₂O → H₃O⁺ + Cl⁻ - H⁺(aq) is shorthand for H₃O⁺(aq) – free H⁺ cannot exist alone in water
Bases in Water
- Bases dissociate to produce hydroxide ions (OH⁻)
Examples:- NaOH(s) → Na⁺(aq) + OH⁻(aq)
- KOH(s) → K⁺(aq) + OH⁻(aq)
- Mg(OH)₂(s) → Mg²⁺(aq) + 2OH⁻(aq)
Soluble bases are called alkalis
Neutralisation at Ionic Level
- Core reaction: H⁺(aq) + OH⁻(aq) → H₂O(l)
- Salt forms from the remaining ions (e.g., Na⁺ + Cl⁻ → NaCl)
Dilution – Important Safety Note
- Dissolving acids/bases in water is highly exothermic (releases heat)
- Always add acid/base slowly to water with stirring
❌ Never add water to concentrated acid/base → risk of splashing, burns, or container breakage - Dilution = mixing with water → lowers concentration of H₃O⁺ or OH⁻ ions per unit volume
Key Takeaway
- Water enables ionisation of acids (→ H₃O⁺) and bases (→ OH⁻)
- These ions define acidic/basic behaviour and drive neutralisation
- Handle concentrated acids/bases with care during dilution
How Strong Are Acid or Base Solutions?
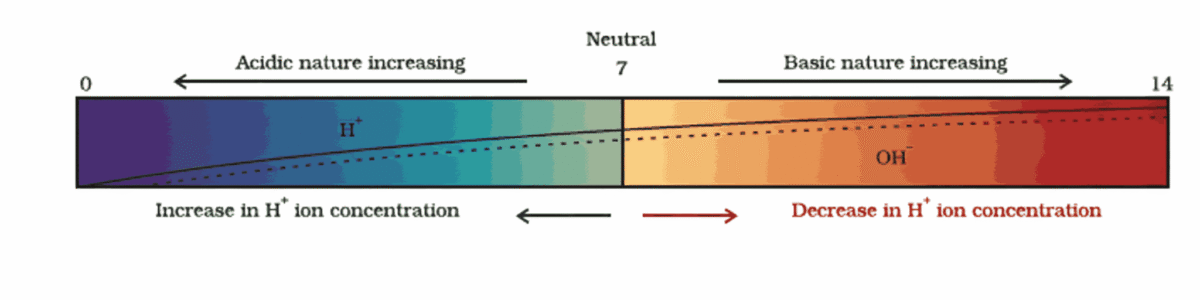
pH Scale – Measuring Acidity/Basicity
- pH scale ranges from 0 to 14
- pH < 7 → Acidic (lower = stronger acid)
- pH = 7 → Neutral (e.g., pure water)
- pH > 7 → Basic/Alkaline (higher = stronger base)
- pH depends on H₃O⁺ (or H⁺) ion concentration:
Higher H⁺ → Lower pH
Higher OH⁻ → Higher pH
Universal Indicator
- A mixture of several indicators
- Shows different colours at different pH values
- Used as pH paper for quick testing
Strong vs. Weak Acids
- Strong acid: Fully dissociates in water → more H⁺ ions (e.g., HCl, HNO₃, H₂SO₄)
- Weak acid: Partially dissociates → fewer H⁺ ions (e.g., CH₃COOH, citric acid)
Strong vs. Weak Bases
- Strong base: Fully dissociates → more OH⁻ ions (e.g., NaOH, KOH)
- Weak base: Partially dissociates → fewer OH⁻ ions (e.g., NH₄OH, Mg(OH)₂)
Key Points
- Strength ≠ Concentration:
- Strength = how completely it ionises
- Concentration = how much acid/base is dissolved
- Same concentration of HCl and acetic acid → HCl is stronger (more H⁺)
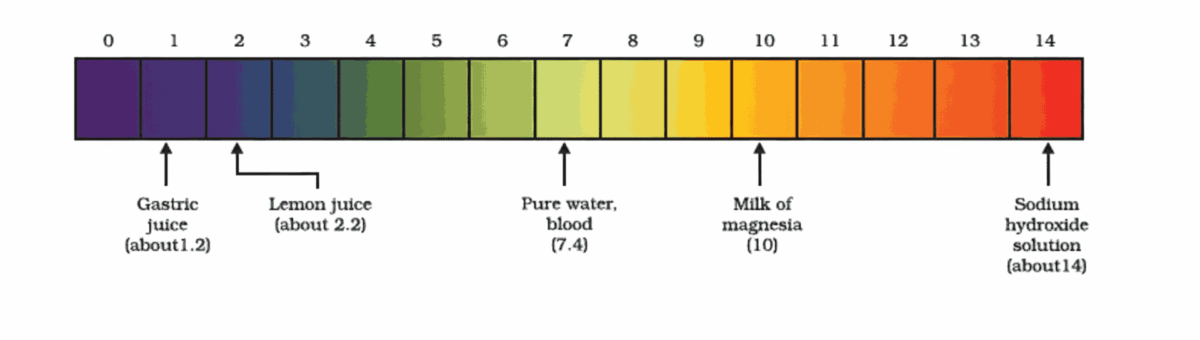
Importance of pH in Everyday Life
pH and Living Organisms
- Human body functions best in pH 7.0 – 7.8
- Even small pH changes can disrupt biological processes
- Acid rain (pH < 5.6) lowers river pH → harms aquatic life
Soil pH and Plant Growth
- Each plant needs a specific soil pH to grow well
- Test soil pH using pH paper or kits
- Match plants to soil pH (e.g., blueberries prefer acidic soil; lavender prefers alkaline)
Human Digestion and pH
- Stomach produces HCl (pH ~1.5–3.5) for digestion
- Excess acid → indigestion, pain
- Antacids (e.g., milk of magnesia – Mg(OH)₂) neutralize excess acid
Tooth Decay and pH
- Enamel (calcium hydroxyapatite) starts dissolving when mouth pH < 5.5
- Mouth bacteria break down sugars → produce acids
- Prevention:
- Brush after meals
- Use basic toothpastes to neutralize acid
Self-Defence in Nature
- Bee sting: Injects methanoic acid → apply baking soda (base) for relief
- Nettle leaves: Release methanoic acid → causes burning sensation
Common Natural Acids
| Source | Acid |
|---|---|
| Vinegar | Acetic acid |
| Citrus fruits | Citric acid |
| Sour milk | Lactic acid |
| Tamarind | Tartaric acid |
| Tomato | Oxalic acid |
| Ant/Nettle sting | Methanoic acid |
More About Salts
Nature of Salts Based on pH
- Strong acid + Strong base → Neutral salt (pH = 7)
Example: NaCl (from HCl + NaOH) - Strong acid + Weak base → Acidic salt (pH < 7)
Example: NH₄Cl - Weak acid + Strong base → Basic salt (pH > 7)
Example: CH₃COONa (sodium acetate)
Common Salt (Sodium Chloride – NaCl)
- Formed by neutralisation: HCl + NaOH → NaCl + H₂O
- Found in seawater and as rock salt (mined from ancient dried seas)
- Rock salt may appear brown due to impurities
- Historically significant – symbol of resistance in Gandhi’s Dandi March
Common Salt as a Raw Material
NaCl is used to manufacture many
everyday chemicals:
- Sodium hydroxide (NaOH) – used in soap, paper, textiles
- Baking soda (NaHCO₃) – cooking, antacids, fire extinguishers
- Washing soda (Na₂CO₃·10H₂O) – cleaning, water softening
- Bleaching powder (CaOCl₂) – disinfectant, whitening agent
Key Takeaway
- Not all salts are neutral – pH depends on the strength of parent acid and base
- Common salt is a key industrial chemical – starting point for making many useful products
Sodium Hydroxide
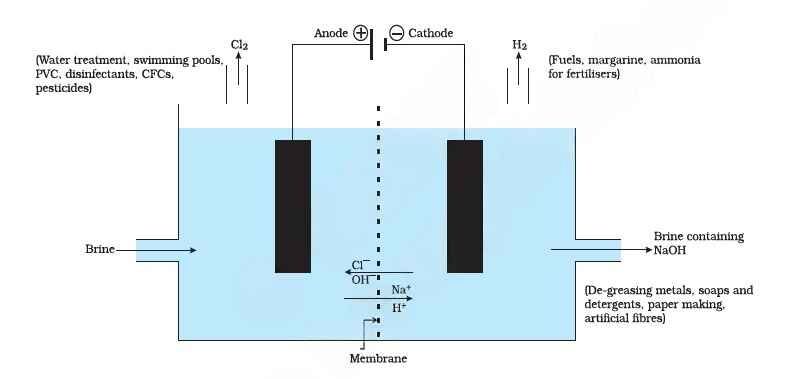
Production: Chlor-Alkali Process
- Raw material: Aqueous sodium chloride (brine)
- Reaction:
2NaCl(aq) + 2H₂O(l) → 2NaOH(aq) + Cl₂(g) + H₂(g) - Process: Electrolysis of brine
- Anode: Chlorine gas (Cl₂) released
- Cathode: Hydrogen gas (H₂) released
- Solution near cathode: Sodium hydroxide (NaOH) forms
Why “Chlor-Alkali”?
- “Chlor” = chlorine
- “Alkali” = sodium hydroxide (a strong base)
Uses of the Three Products
- Sodium hydroxide (NaOH):
- Soap, paper, and rayon production
- Petroleum refining
- Drain cleaners
- Chlorine (Cl₂):
- Water purification
- PVC, disinfectants, and solvents
- Hydrogen (H₂):
- Fuel, ammonia (fertiliser) production
- Hydrogenation of oils
Bleaching Powder
Preparation
- Made by reacting chlorine gas (from chlor-alkali process) with dry slaked lime [Ca(OH)₂]
- Chemical equation:
Ca(OH)₂ + Cl₂ → CaOCl₂ + H₂O - Actual composition is complex, but commonly represented as CaOCl₂
Key Uses
- Bleaching:
- Cotton and linen in textile industry
- Wood pulp in paper factories
- Washed clothes in laundries
- Oxidising Agent:
- Used in various chemical manufacturing processes
- Disinfectant:
- Purifies drinking water by killing germs and bacteria
Important Note
- Releases chlorine when mixed with water or acid → responsible for bleaching and disinfecting action
Key Takeaway
- Bleaching powder is a versatile chemical derived from chlorine and lime
- Essential for cleaning, whitening, and sanitation in homes and industries
Baking Soda
Chemical Identity
- Chemical name: Sodium hydrogencarbonate
- Formula: NaHCO₃
- Nature: Mild, non-corrosive basic salt (pH > 7)
- Raw material: Made from NaCl, CO₂, NH₃, and H₂O
NaCl + H₂O + CO₂ + NH₃ → NH₄Cl + NaHCO₃
Reaction on Heating
- Decomposes to release CO₂ gas:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂↑ - This makes food crispy, soft, and spongy
Key Uses
1. In Cooking
- Used in pakoras for crispiness
- Main ingredient in baking powder (mixed with mild edible acid like tartaric acid)
- When wet or heated:
NaHCO₃ + H⁺ → CO₂↑ + H₂O + sodium salt
→ CO₂ makes cakes and bread rise
2. As an Antacid
- Neutralises excess stomach acid
- Provides quick relief from acidity and heartburn
3. In Fire Safety
- Used in soda-acid fire extinguishers
- CO₂ released smothers fire by cutting off oxygen
Washing Soda
Chemical Identity
- Chemical name: Sodium carbonate decahydrate
- Formula: Na₂CO₃·10H₂O
- Preparation:
- First, baking soda is heated to form anhydrous sodium carbonate:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂ - Then, recrystallisation with water gives washing soda:
Na₂CO₃ + 10H₂O → Na₂CO₃·10H₂O
- First, baking soda is heated to form anhydrous sodium carbonate:
- Nature: Basic salt (pH > 7)
What Does “10H₂O” Mean?
- It means 10 water molecules are chemically bound per formula unit of Na₂CO₃
- These are called water of crystallisation – part of the crystal structure
- Does not make the solid wet; it’s a hydrated crystalline solid
Key Uses
1. Industrial Applications
- Used in glass, soap, and paper manufacturing
- Raw material for making other sodium compounds like borax
2. Household Cleaning
- Effective cleaning agent for surfaces, utensils, and stains
3. Water Softening
- Removes permanent hardness of water by precipitating calcium and magnesium ions
Are the Crystals of Salts Really Dry?
Water of Crystallisation
- Fixed number of water molecules chemically bound in one formula unit of a salt
- Makes crystals appear dry but still contain water
- Not “wet” – water is part of the crystal structure, not free liquid
Example:
Copper Sulphate
- Hydrated form: CuSO₄·5H₂O (blue crystals)
- On heating: loses water → becomes anhydrous CuSO₄ (white powder)
- Add water again → blue colour returns (rehydration)
Other Hydrated Salts
- Washing soda: Na₂CO₃·10H₂O → 10 water molecules
- Gypsum: CaSO₄·2H₂O → 2 water molecules
Key Insight
- Na₂CO₃·10H₂O is not wet – the “10H₂O” is water of crystallisation, not surface moisture
- These water molecules give salts their shape, colour, and stability
Practical Note
- Heating removes water → changes colour and texture
- Rehydration may restore original form (as in copper sulphate)
Plaster of Paris
What Is It?
- Chemical name: Calcium sulphate hemihydrate
- Formula: CaSO₄·½H₂O
- Made by heating gypsum (CaSO₄·2H₂O) at 373 K
CaSO₄·2H₂O → CaSO₄·½H₂O + 1½H₂O
Why “½H₂O”?
- The “½” means two CaSO₄ units share one water molecule
- It’s a simplified way to show the average water content per formula unit
Key Property: Setting Action
- When mixed with water, it reforms gypsum:
CaSO₄·½H₂O + 1½H₂O → CaSO₄·2H₂O - Forms a hard, solid mass within minutes → ideal for casts and molds
Common Uses
- Medical: Casts for fractured bones
- Art & Decoration: Making toys, statues, and ornamental designs
- Construction: Smoothing walls and ceilings (“plastering”)
Why “Plaster of Paris”?
- Named after Paris (France), where large deposits of gypsum were found and used historically for construction and plaster
Key Takeaway
- Plaster of Paris is a hemihydrate that hardens with water
- Its reversible reaction with water makes it versatile in medicine, art, and building
- Despite the odd “½H₂O” formula, it reflects real crystal structure—not literal half molecules
FAQs – Acids Bases And Salts Short Notes Class 10
Q1. What makes a substance acidic or basic?
A: Acids release H⁺ (or H₃O⁺) ions in water, while bases release OH⁻ ions. These ions determine their chemical behaviour.
Q2. Are all sour things acids and all bitter things bases?
A: Generally yes—but never taste lab chemicals! We use indicators like litmus or turmeric to test safely.
Q3. Why do we need to learn about pH?
A: pH affects digestion, tooth health, soil fertility, water quality, and even rain—it’s vital in daily life .
Q4. Why is NaCl neutral, but NH₄Cl acidic?
A: NaCl comes from a strong acid (HCl) + strong base (NaOH) → neutral.
NH₄Cl comes from strong acid (HCl) + weak base (NH₄OH) → acidic salt.
Q5. Can bases react with metals like acids do?
A: Yes—but only amphoteric metals like Zn or Al react with strong bases (e.g., NaOH) to produce H₂ gas.
Q6. Why does CO₂ turn lime water milky, but the milkiness disappears with excess CO₂?
A:
- First: CO₂ + Ca(OH)₂ → CaCO₃ (white ppt) → milky
- Excess CO₂: CaCO₃ + CO₂ + H₂O → Ca(HCO₃)₂ (soluble) → clear again
Q7. What’s the difference between baking soda and washing soda?
A:
- Baking soda: NaHCO₃ – mild base, used in cooking & antacids
- Washing soda: Na₂CO₃·10H₂O – stronger base, used for cleaning & water softening
- You can make washing soda by heating baking soda .
Q8. Are hydrated salts wet?
A: No! The water (e.g., 5H₂O in CuSO₄·5H₂O) is chemically bound in the crystal—called water of crystallisation. It’s dry to touch.
Q9. Why is Plaster of Paris called “Paris”?
A: It’s named after large gypsum deposits in Montmartre, Paris, historically used to make it.
Q10. Why must acid be added to water—not water to acid—during dilution?
A: Adding water to concentrated acid causes sudden, violent heat release, risking splashes, burns, or glass breakage. Always add acid slowly to water with stirring.
Q11. How does pH affect tooth decay?
A: Enamel dissolves when mouth pH drops below 5.5 due to acid from bacteria. Basic toothpastes neutralise acid and prevent decay.
Q12. Can I use baking soda for bee stings?
A: Yes! Bee venom is acidic (methanoic acid). A mild base like baking soda neutralises it and reduces pain .
Q13. Is rainwater always neutral?
A: No! Normal rain is slightly acidic (pH ~5.6). Acid rain (pH < 5.6) harms aquatic life and buildings.
Q14. Why do farmers test soil pH?
A: Plants need specific pH ranges. Too acidic/alkaline soil blocks nutrient absorption—farmers add lime (to reduce acidity) or compost to adjust pH .
Q15. Are all salts edible?
A: No! Only sodium chloride (table salt) is safe to eat. Others like copper sulphate or lead salts are toxic.
Q16. How do antacids work?
A: They contain mild bases (e.g., Mg(OH)₂) that neutralise excess stomach acid, relieving indigestion




