Struggling to remember long textbook paragraphs? Confused by complex definitions before your CBSE test? We have the solution! “Atoms And Molecules Short Notes Class 9” is sourced from NCERT textbook chapter 3.
These ultra-short, high-yield notes are designed specifically for Class 9 CBSE students who want to:
✅ Memorise key facts without overload
✅ Retain information longer with bolded keywords, bite-sized points, and instant self-checks
Every line sticks to your NCERT syllabus— no extras, just what you need to score. From Dalton’s theory to writing chemical formulae, each topic is broken into scannable blocks with Quick Check questions to lock learning in place.
Use these notes for last-minute revision, daily recall, or to build confidence before exams.
Atoms And Molecules Short Notes Class 9 = Your shortcut to clarity, retention, and top marks.
Introduction
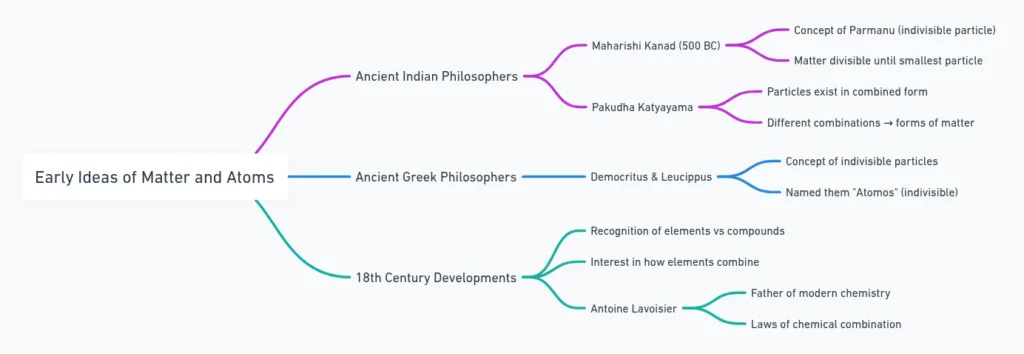
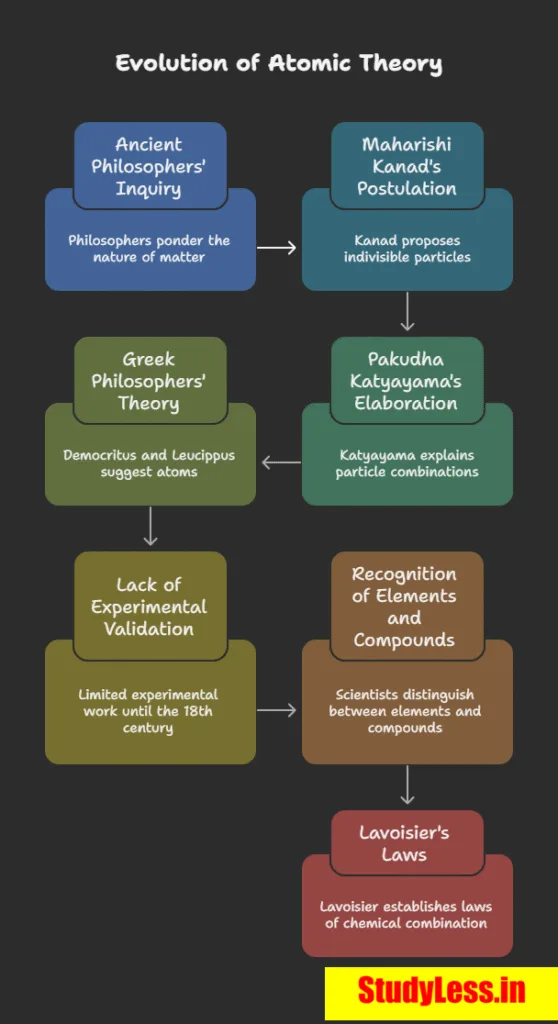
Maharishi Kanad (India, ~500 BC):
✔Matter divisible → smallest particle = Parmanu (indivisible).
Pakudha Katyayama:
✔Parmanu combine → form all matter.
Democritus & Leucippus (Greece):
Indivisible particles = atoms (atomos = uncuttable).
Lavoisier → founded chemical science with two laws of chemical combination.
Quick Check Questions
- Who coined Parmanu? What does it mean?
- What did Greeks call indivisible particles?
- Why weren’t ancient theories scientific?
- What did Lavoisier establish?
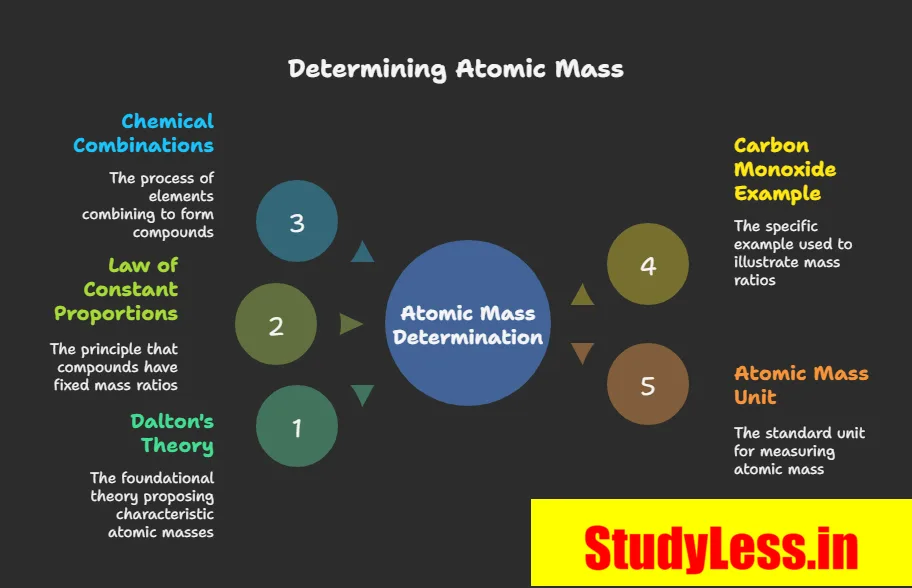
Laws of Chemical Combination
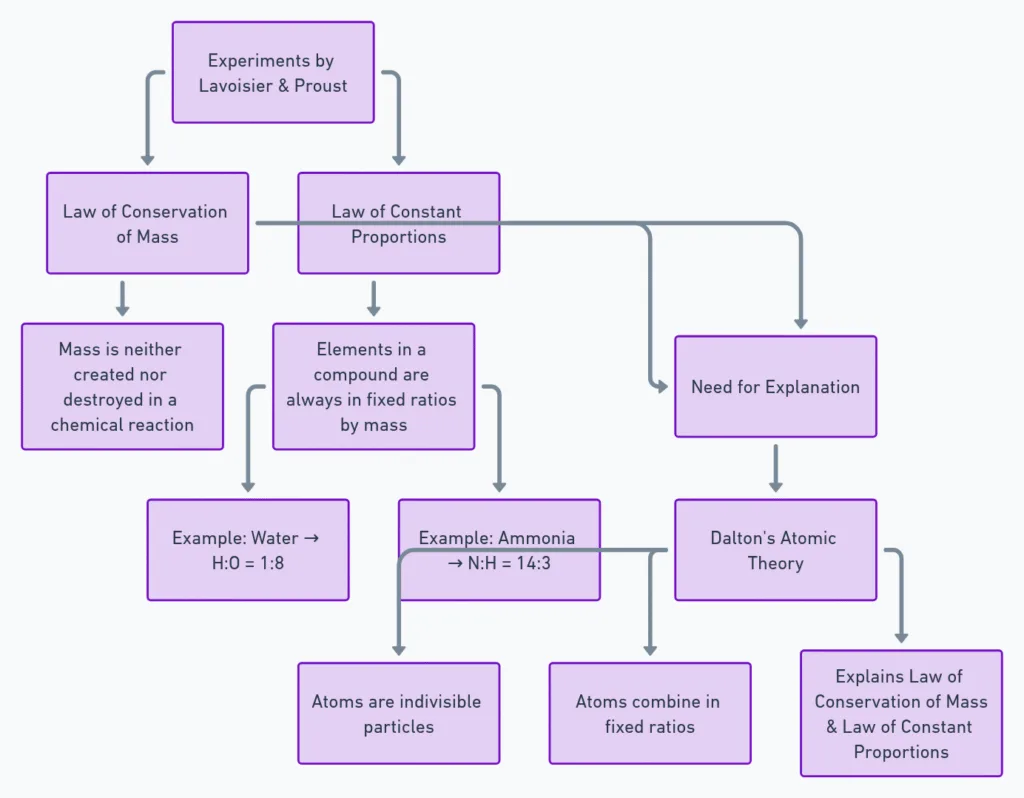
- Law of Conservation of Mass (By Lavoisier):
- Mass is neither created nor destroyed in a chemical reaction.
- Mass is neither created nor destroyed in a chemical reaction.
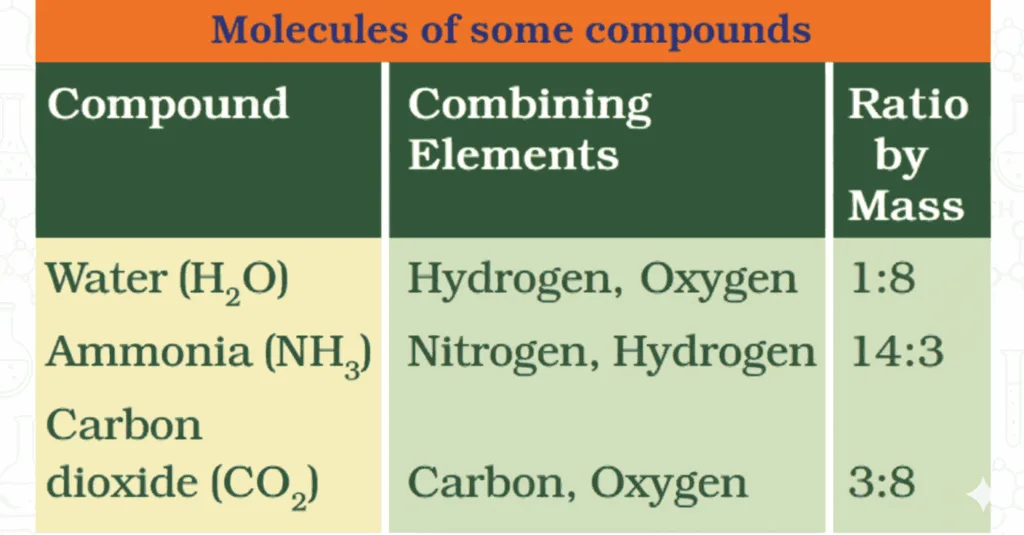
- Law of Constant Proportions (By Proust):
- In a compound, elements always combine in fixed mass ratios.
- E.g., Water = H:O = 1:8; Ammonia = N:H = 14:3.
- Also called Law of Definite Proportions.
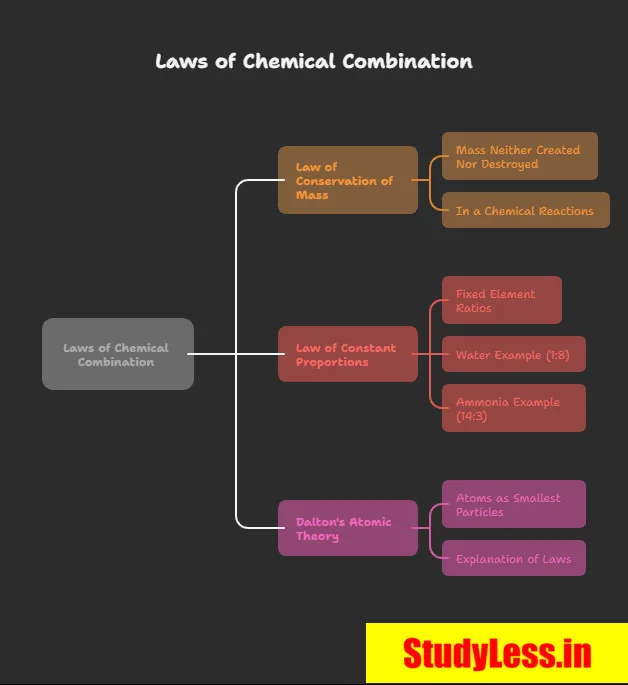
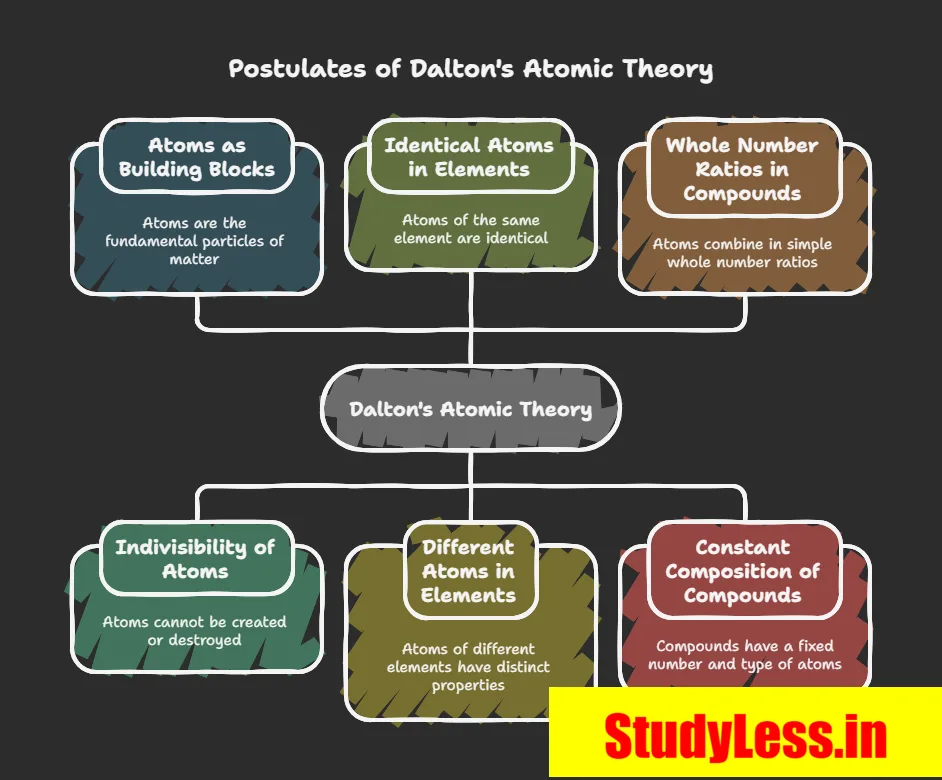
Dalton’s Atomic Theory:
- Explained both laws.
- Matter made of atoms (indivisible particles).
- Revived Greek term “atom” with scientific basis.
Also Read | Short Notes Force And Laws Of Motion Class 9,With Infographics, Easy To Memorize
Quick Check Questions
- What does the Law of Conservation of Mass state?
- Give the mass ratio of H:O in water and N:H in ammonia.
- Who proposed the Law of Constant Proportions?
- How did Dalton’s theory support these laws?
Dalton’s Atomic Theory
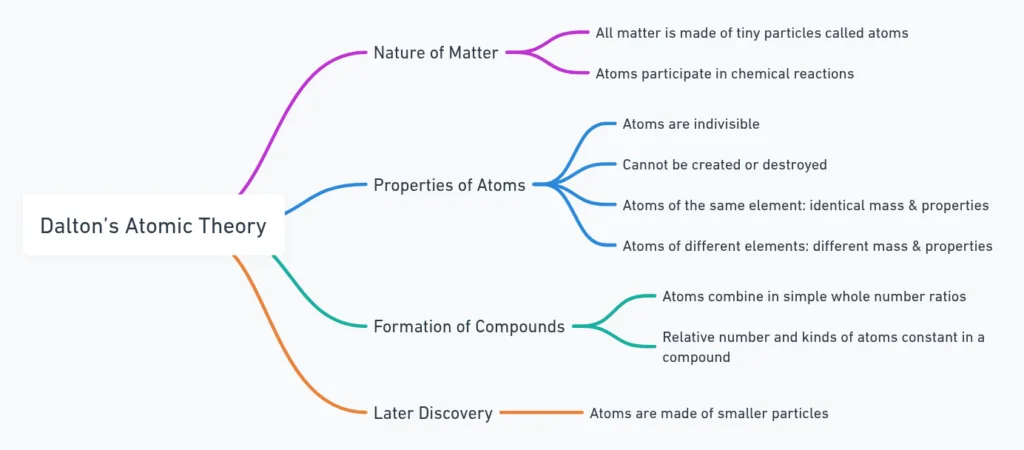
All matter made of atoms (tiny, indivisible).
Key postulates
- Atoms take part in reactions.
- Not created/destroyed → explains conservation of mass.
- Same element → atoms identical in mass & properties.
- Different elements → atoms different.
- Combine in small whole-number ratios.
- Compounds = fixed atom ratio → explains constant proportions.
- Later: atoms are divisible (subatomic particles exist).
Quick Check
- Which postulate supports mass conservation?
- Why do compounds have fixed composition?
- Are atoms really indivisible?
- How does the theory justify the Law of Constant Proportions?
What is an Atom?
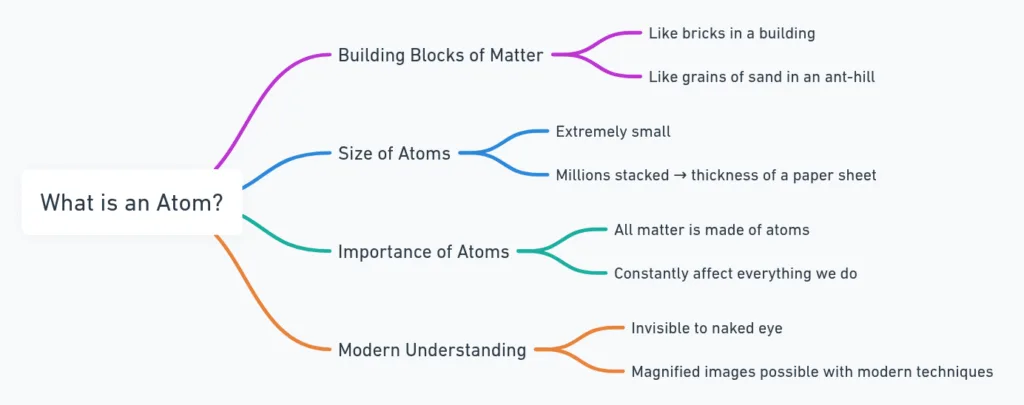
- An atom = building block of all matter.
- Extremely small:
- Millions stacked ≈ thickness of one sheet of paper.
- Invisible, but everything is made of atoms.
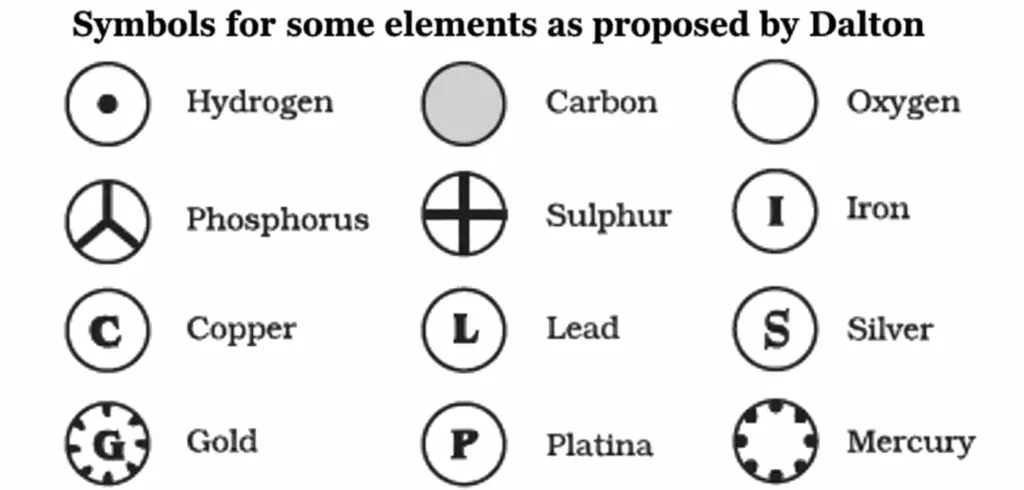
Dalton
Related | Atoms and Molecules MCQ Quiz
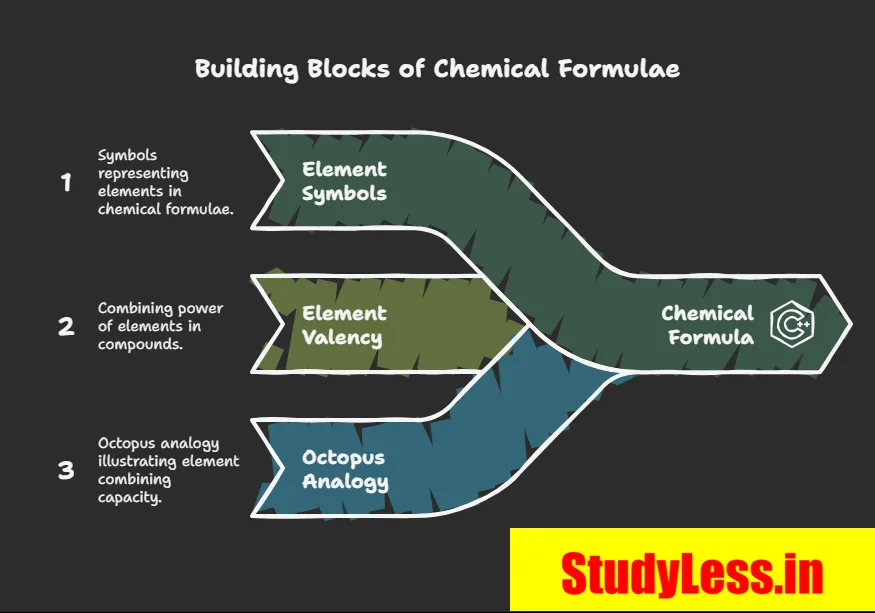
Modern Symbols of Elements
- Dalton: First to use element symbols = 1 atom of that element.
- Berzelius: Proposed symbols from 1–2 letters of element’s name.
- IUPAC: Now approves all names & symbols and Units
Quick Check
- Why are atoms important if we can’t see them?
- How small are atoms? Give a comparison.
- Can we observe atoms today? How?
Rules for symbols
- 1st letter = uppercase, 2nd = lowercase → e.g., Al, Co (not AL, CO).
- Usually from English name:
- H (hydrogen), Cl (chlorine), Zn (zinc).
- Some from Latin/Greek/German:
- Fe (iron ← ferrum), Na (sodium ← natrium), K (potassium ← kalium).
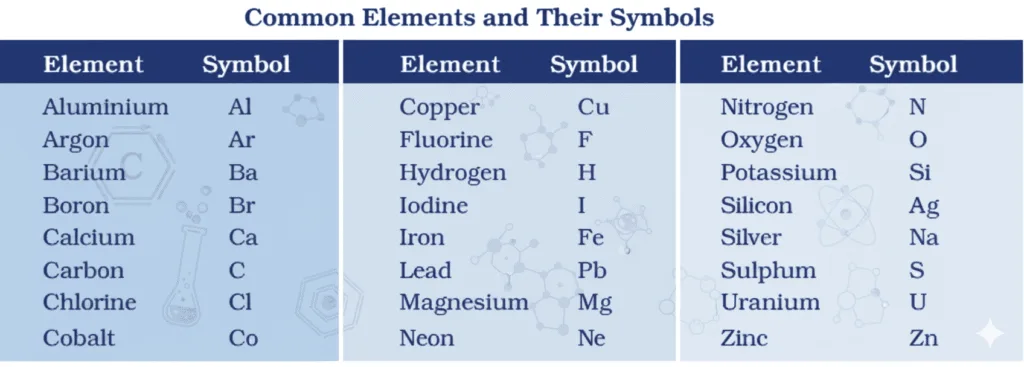
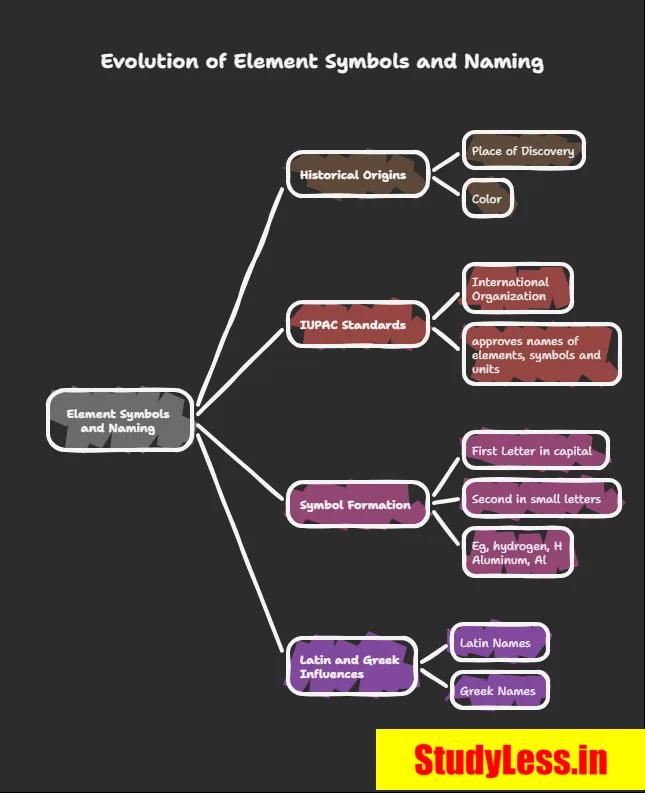
→ Each element = unique name + symbol.
Quick Check
- Who introduced modern element symbols?
- Why is Co correct but CO wrong for cobalt?
- Give symbols for iron, sodium, and potassium—and their Latin origins.
- What are the IUPAC symbol rules for capitalization?
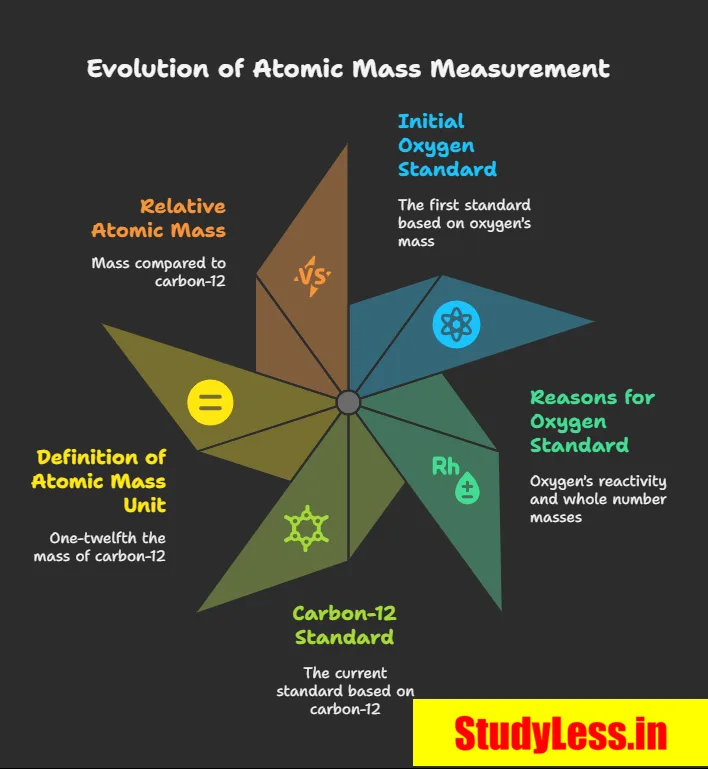
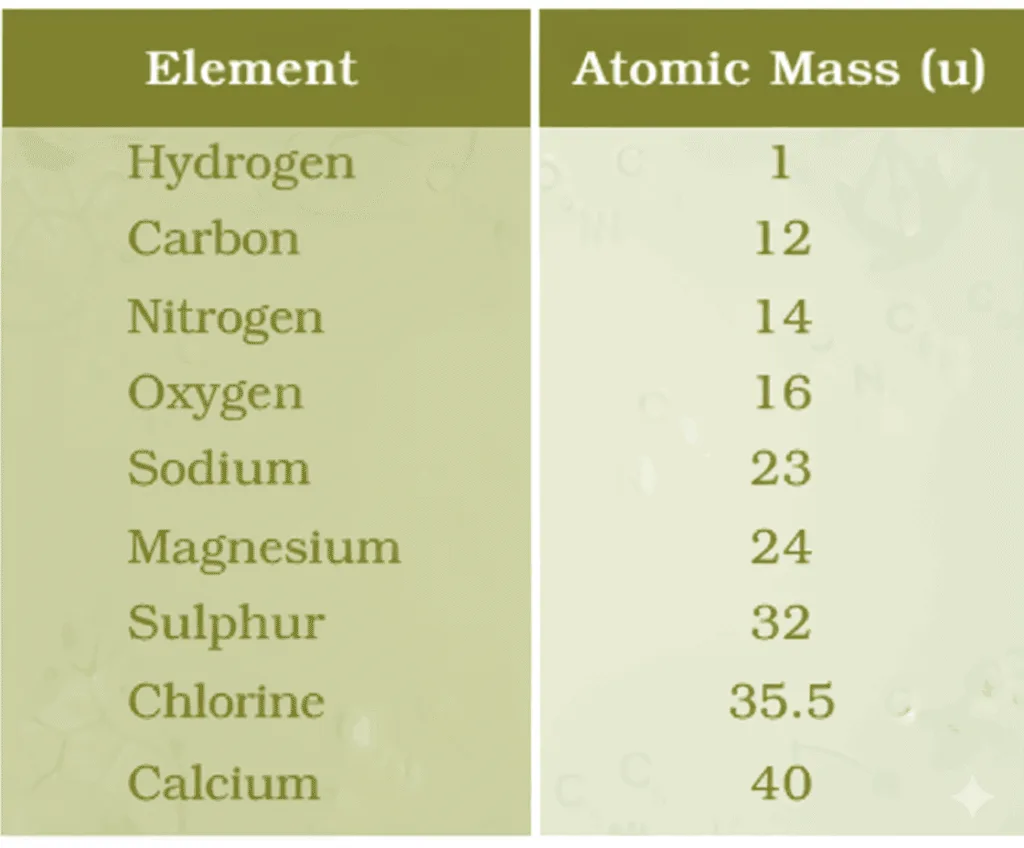
Atomic Mass
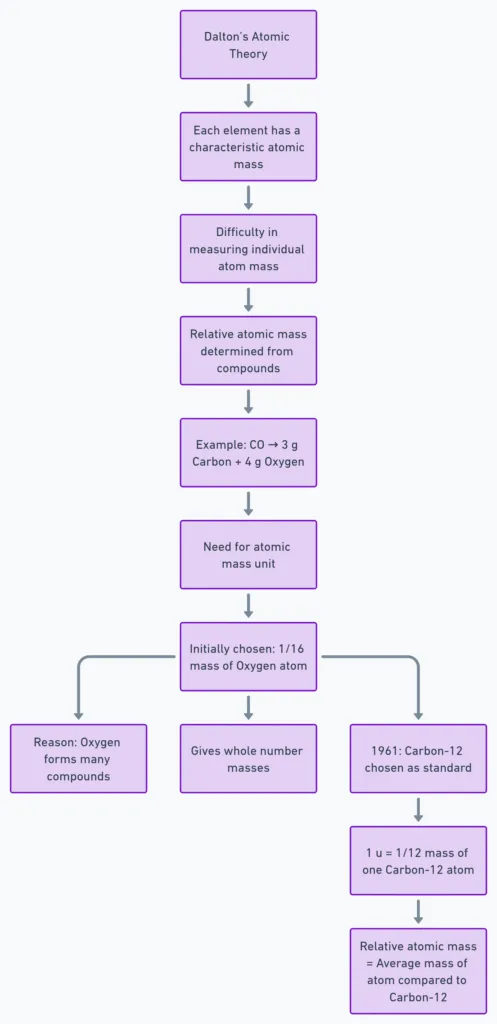
- Dalton: Each element has a characteristic atomic mass → explained law of constant proportions.
- Actual atom mass too small therefore used relative atomic masses via compound ratios.
- E.g., CO: 3 g C + 4 g O → O = 4/3 × mass of C.
- Early unit: 1/16 mass of natural oxygen atom — chosen because:
• Oxygen forms compounds with many elements.
• Gave near-whole-number masses for most elements. - Since 1961: Standard = carbon-12 isotope.
- 1 u = 1/12th mass of one carbon-12 atom.
- Relative atomic mass = average atom mass relative to 1 u.
Quick Check
- Why did scientists switch from actual to relative atomic masses?
- What mass ratio of C:O is seen in carbon monoxide?
- Give two reasons oxygen was initially used as the standard.
- What is the modern definition of 1 u?
- How is relative atomic mass defined today?
- What are the two reasons for oxygen-based unit?
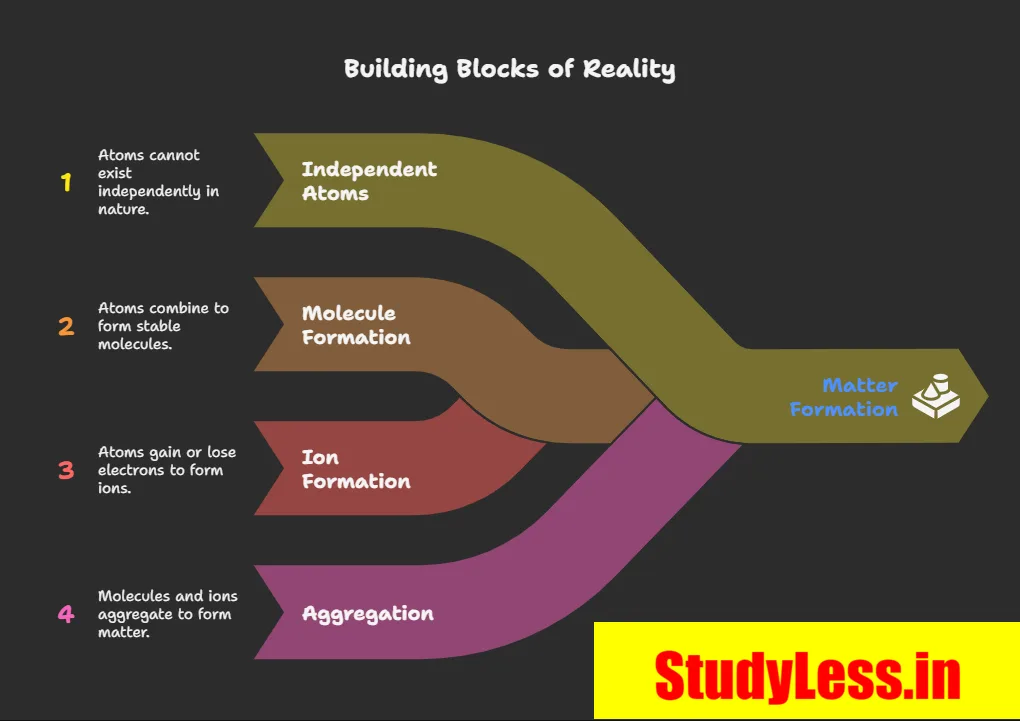
How Do Atoms Exist?
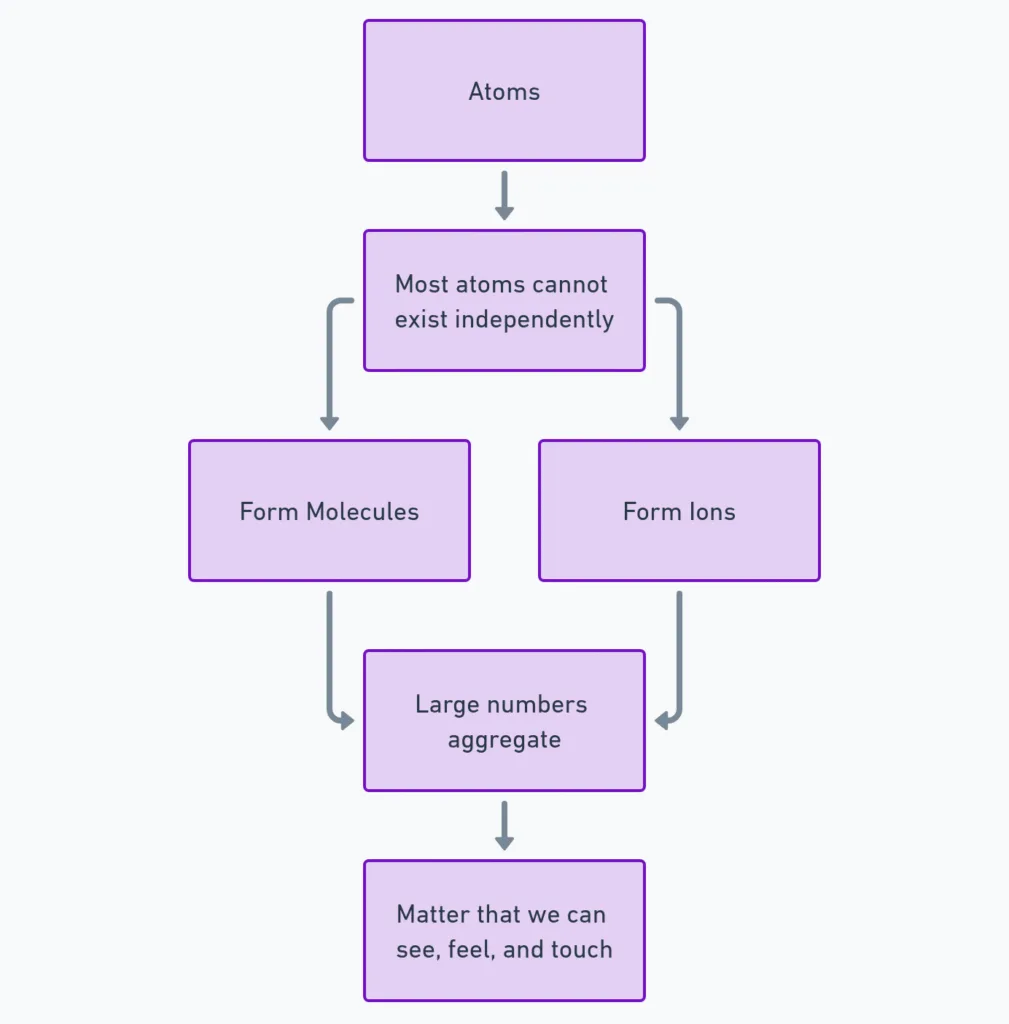
- Most atoms cannot exist independently.
- They form molecules or ions.
- These aggregate in large numbers → form visible/tangible matter.
Quick Check
- Can most atoms exist alone?
- What do atoms form instead?
- How does visible matter form from atoms?
What is a Molecule?
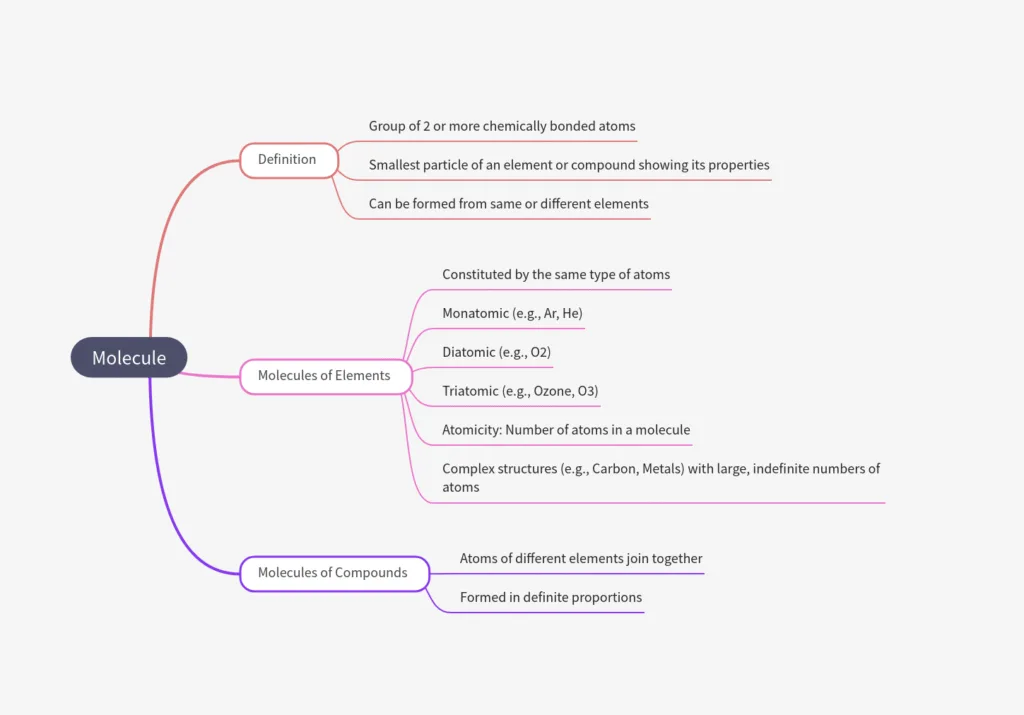
- Molecule = 2 or more atoms chemically bonded (tightly held by attractive forces).
- Smallest particle of an element or compound that:
• Can exist independently,
• Shows all properties of the substance. - Formed by atoms of same or different elements.
Molecules of Elements
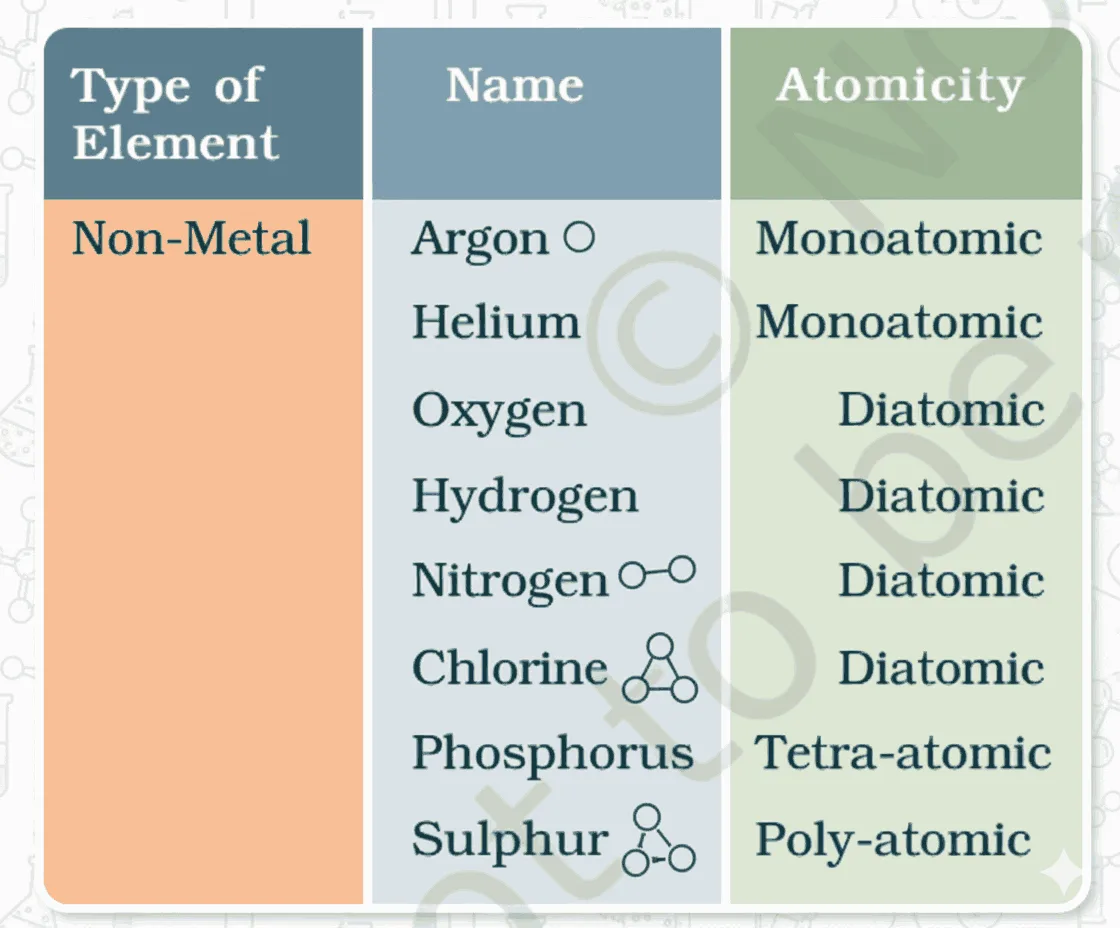
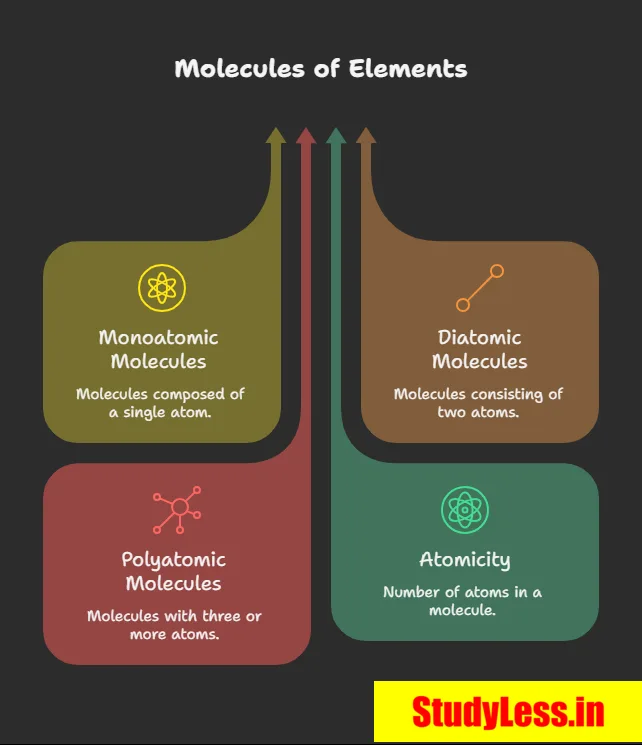
- Molecules of an element = made of same-type atoms.
- Noble gases (e.g., Ar, He) = monoatomic (1 atom/molecule).
- Most non metals:
- O₂ = diatomic (2 atoms),
- O₃ (ozone) = triatomic (3 atoms).
- Atomicity = number of atoms in a molecule.
- Metals & carbon:
- No simple molecules → very large, indefinite number of bonded atoms.
Quick Check
- What is a molecule made of?
- Can a molecule exist independently?
- Can molecules form from same or different elements? Give one example of each.
Quick Check
- What is atomicity?
- Give examples of monoatomic, diatomic, and triatomic molecules of elements.
- Why don’t metals form simple molecules like O₂?
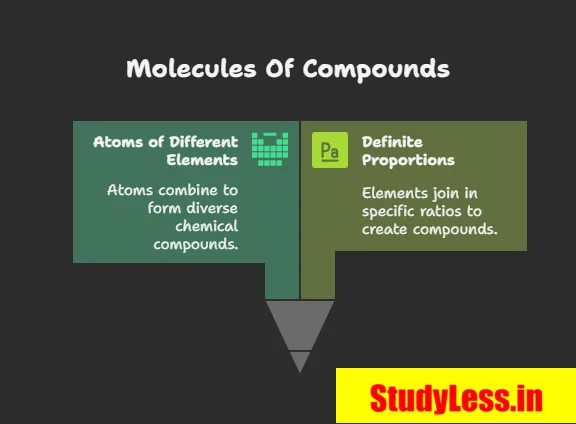
Molecules of Compounds
- Formed when atoms of different elements join in definite proportions.
- Represent the smallest unit of a compound that retains its chemical properties.
Quick Check
- What are molecules of compounds made of?
- In what ratio do atoms combine to form compounds?
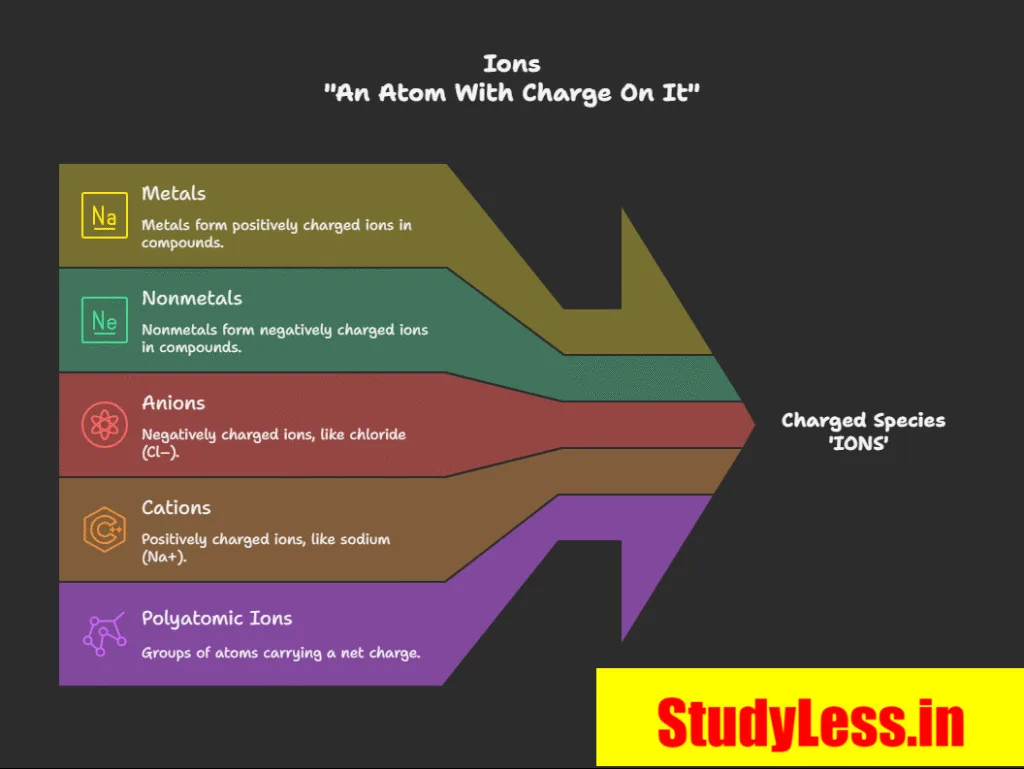
What is an Ion?
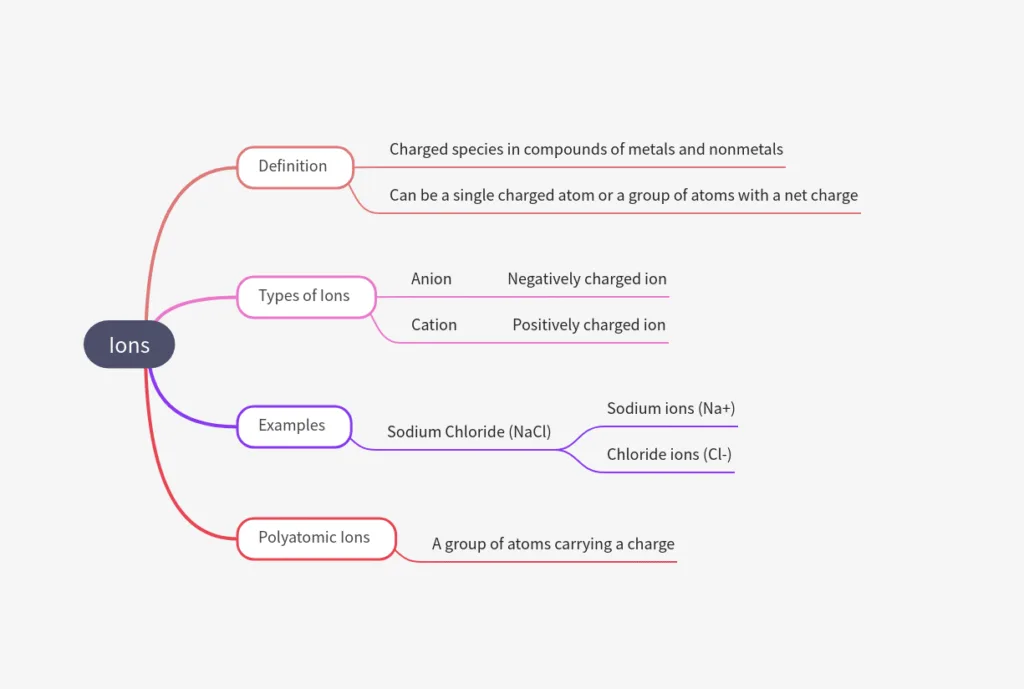
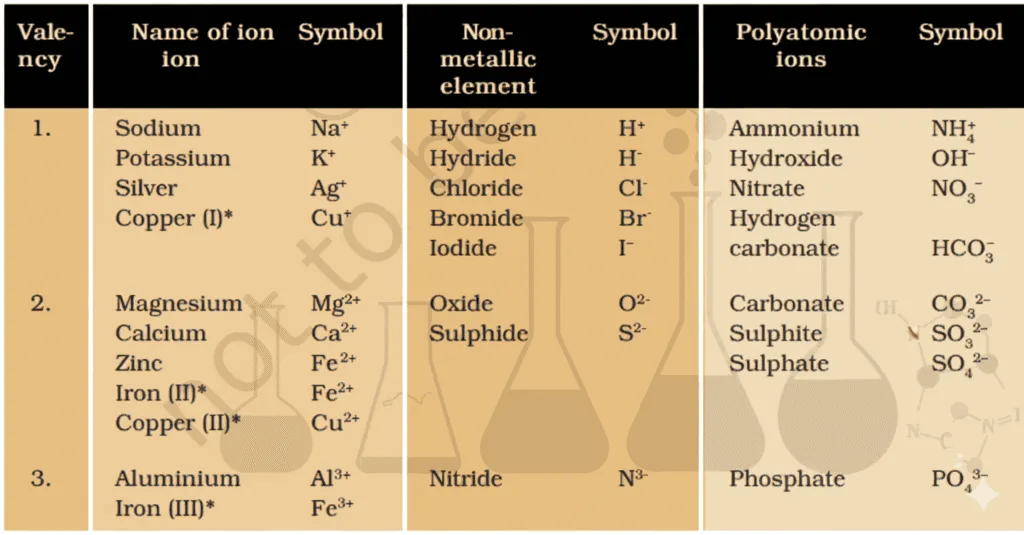
- Ion = charged species in compounds of metals + nonmetals.
- Can be:
• Single atom (e.g., Na+, Cl–)
• Group of atoms with net charge → polyatomic ion. Eg, NH+ (Ammonium ion) - Cation = positively charged ion (e.g., Na+).
- Anion = negatively charged ion (e.g., Cl–).
- Example: NaCl = Na+ (cation) + Cl– (anion).
Quick Check
- What is an ion?
- Differentiate cation and anion.
- What is a polyatomic ion? Give an example (not from text if possible).
Writing Chemical Formulae
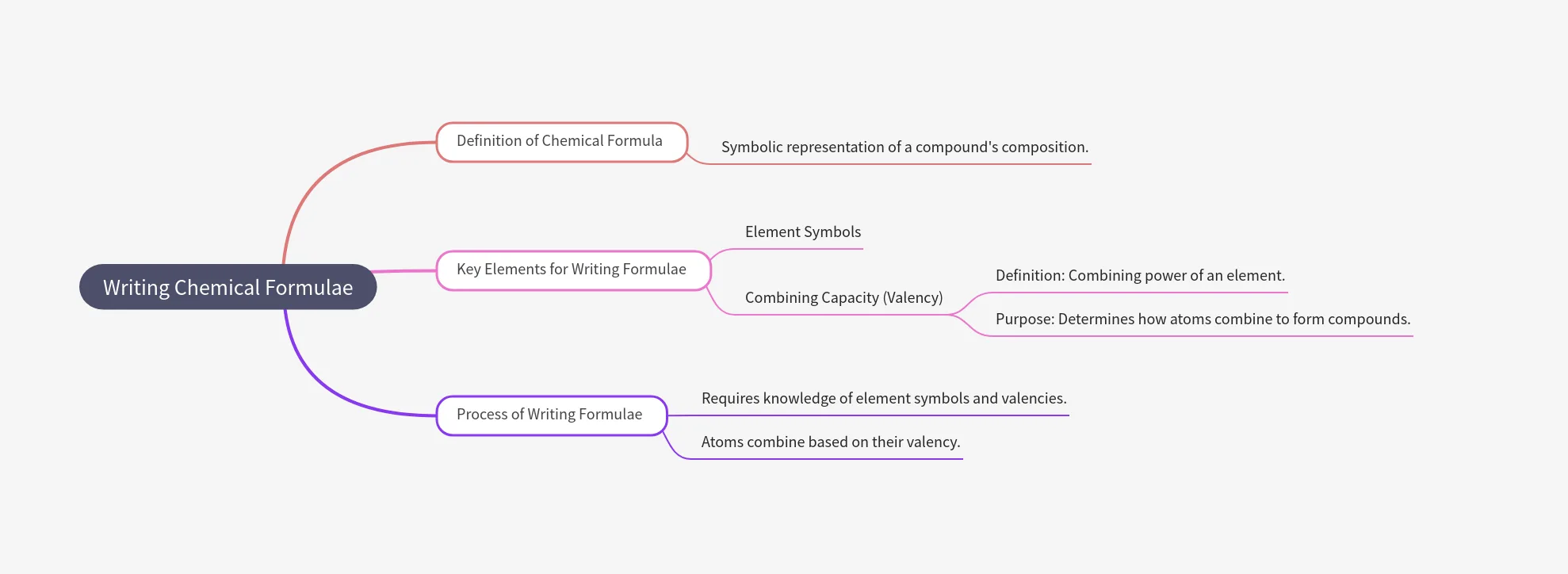
- Chemical formula = symbolic representation of a compound’s composition.
- Requires knowledge of symbols and valency (combining capacity).
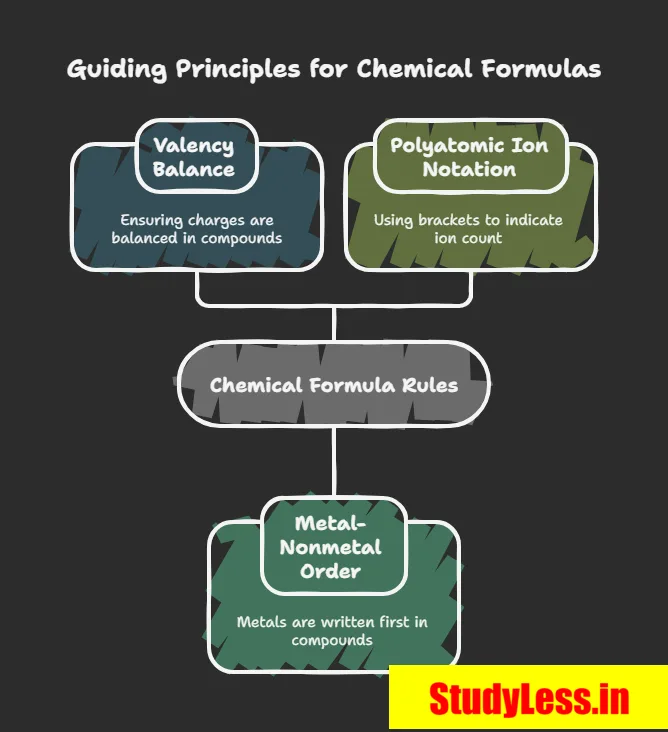
Rules:
- Valencies/charges must balance.
- Metal first, then non-metal:
- E.g., CaO, NaCl, FeS, CuO.
- For polyatomic ions:
✔Use brackets if more than one ion: Mg(OH)₂.
✔No brackets if only one ion: NaOH.
Quick Check
- What is valency?
- Why is Ca written before O in CaO?
- When do you use brackets in a formula? Give an example.
- Write the formula for sodium hydroxide and magnesium nitrate.
Formulae of Simple Compounds
- Binary compounds: made of two different elements.
- Use valency (or ion charge) to write formulae.
- Criss-cross method: swap valencies as subscripts → balance charges.
- Final formula must be electrically neutral; charges not shown.
Key Rules:
- Metal first, then non-metal (e.g., MgCl₂, CaO).
- Simplify if subscripts have common factor: Ca₂O₂ → CaO.
- Use brackets for polyatomic ions if more than one:
- Ca(OH)₂ ✓ (not CaOH₂)
- NaOH → no bracket (only one OH⁻).
Examples:
- HCl, H₂S, CCl₄
- MgCl₂ (Mg2+ + 2Cl–)
- Al₂O₃, CaO
- NaNO₃, Ca(OH)₂, Na₂CO₃, (NH₄)₂SO₄
Quick Check
- What is a binary compound?
- How do you use the criss-cross method?
- Why is calcium hydroxide written as Ca(OH)₂ and not CaOH₂?
- Write formulae for:
a) aluminium oxide
b) sodium carbonate
c) ammonium sulphate
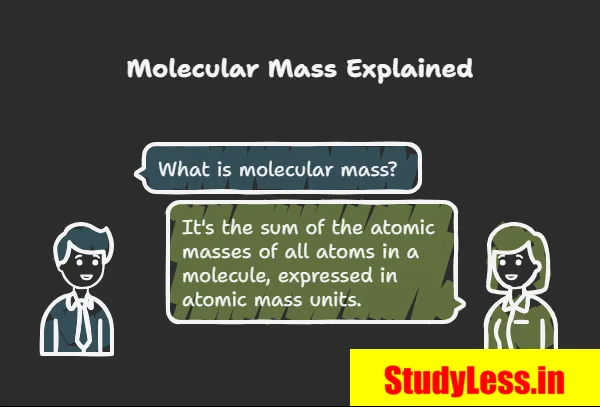
Molecular Mass
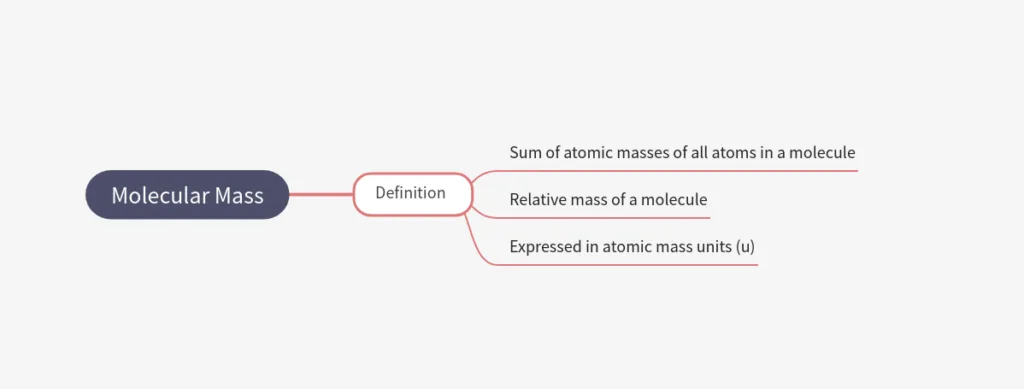
- Molecular mass = sum of atomic masses of all atoms in a molecule.
- Expressed in atomic mass units (u).
- Relative mass of a molecule.
Examples:
- Water (H₂O):
= (2 × H) + (1 × O) = (2 × 1 u) + (1 × 16 u) = 18 u - Nitric acid (HNO₃):
= (1 × H) + (1 × N) + (3 × O) = 1 + 14 + 48 = 63 u
Quick Check
- What is molecular mass?
- Calculate molecular mass of:
a) CO₂
b) NH₃ - Why is molecular mass expressed in u?
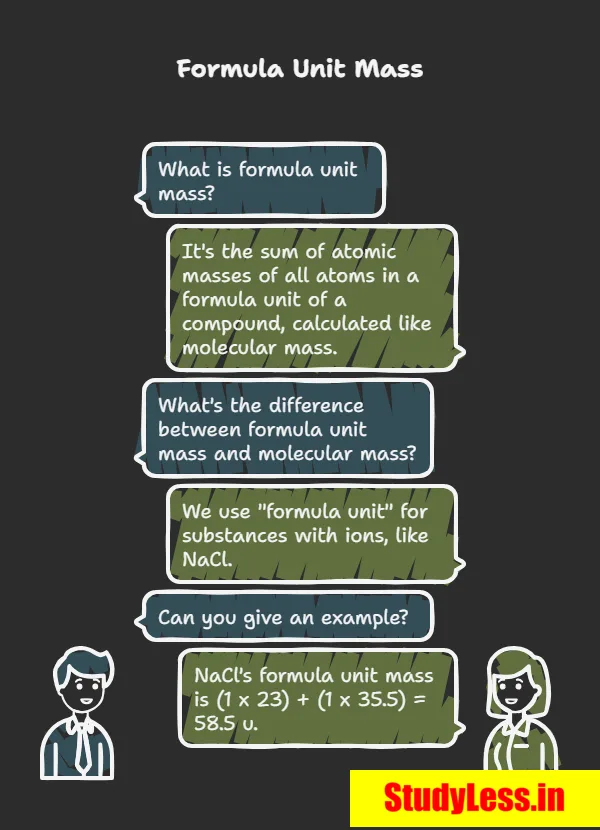
Formula Unit Mass
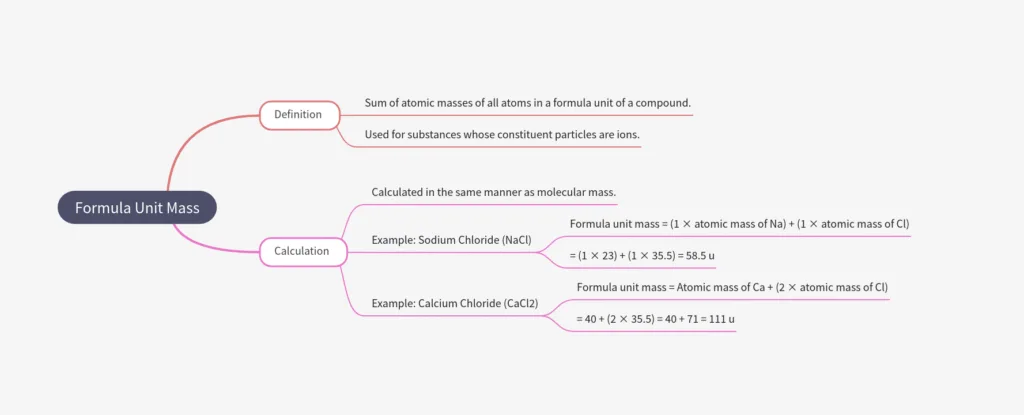
- Formula unit mass = sum of atomic masses of all atoms in a formula unit of an ionic compound.
- Calculated same as molecular mass, but used for ionic substances (made of ions, not molecules).
Examples:
- NaCl: 1×Na + 1×Cl = 23 + 35.5 = 58.5 u
- CaCl₂: 1×Ca + 2×Cl = 40 + 2×35.5 = 111 u
Quick Check
- What is the difference between molecular mass and formula unit mass?
- Why do we use formula unit mass for NaCl but molecular mass for H₂O?
- Calculate formula unit mass of MgO (Mg = 24 u, O = 16 u).
THE END😊
FAQs : Atoms And Molecules Short Notes Class 9
Q1: Are these short notes aligned with the latest CBSE Class 9 Science syllabus?
Yes! These notes strictly follow the NCERT Class 9 Science textbook (Chapter 3: Atoms and Molecules) and cover all key topics—from Dalton’s atomic theory and laws of chemical combination to molecular mass and chemical formulae—as per the current CBSE curriculum.
Q2: Can I rely on these short notes for exam preparation?
Absolutely. Designed for quick revision and strong retention, these notes highlight only the most important concepts, definitions, and examples. Each section ends with self-check questions to test your understanding—perfect for scoring well in school exams and periodic tests.
Q3: How are these notes different from regular textbook content?
Unlike dense textbook paragraphs, these notes are ultra-concise, keyword-bolded, and visually scannable. Complex ideas are broken into bite-sized points, making them easier to read, memorise, and recall—especially during last-minute revision.
Q5: Are these short notes suitable for students who find Chemistry difficult?
Definitely! These notes simplify abstract ideas (like atoms, ions, valency, and polyatomic ions) using plain language, real examples (NaCl, H₂O, O₂), and logical flow—helping even beginners build a strong foundation without stress.



Please prepare mind map for whole unit
Don’t prepare topic wise